14 CLINICAL STUDIES
Two multicenter, randomized, double-blind, parallel-group, vehicle-controlled trials (Trials 1 and 2) treated a total of 1522 subjects 2 to 79 years of age (86.3% of subjects were 2 to 17 years of age) with a 5% to 95% treatable BSA. At baseline, 38.5% of the subjects had an Investigator's Static Global Assessment [ISGA] of mild (2), and 61.5% had an ISGA of moderate (3), in the overall assessment of atopic dermatitis (erythema, induration/papulation, and oozing/crusting) on a severity scale of 0 to 4.
In both trials, subjects were randomized 2:1 to receive EUCRISA or vehicle applied twice daily for 28 days. The primary efficacy endpoint was the proportion of subjects at Day 29 who achieved success, defined as an ISGA grade of clear (0) or almost clear (1) with a 2-grade or greater improvement from baseline, comparing EUCRISA-treated subjects to vehicle-treated subjects.
Efficacy results from the two trials are summarized in Table 2.
Table 2: Primary Efficacy Outcomes in Subjects with Mild to Moderate Atopic Dermatitis at Day 29 | Trial 1 | Trial 2 |
|---|
EUCRISA
Twice Daily
(N=503) | Vehicle
Twice Daily
(N=256) | EUCRISA
Twice Daily
(N=513) | Vehicle
Twice Daily
(N=250) |
|---|
|
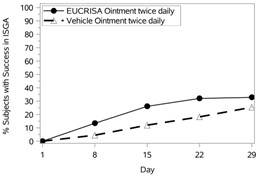
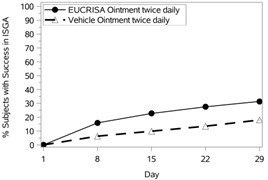
Success in ISGA* | 32.8% | 25.4% | 31.4% | 18.0% |
The success rates over time are presented in Figure 1.
|
Figure 1: Success in ISGA* Over Time in Subjects with Mild to Moderate Atopic Dermatitis |
Trial 1 | Trial 2 |
 |  |
One randomized, double-blind, vehicle-controlled trial (Trial 3) assessed the efficacy and safety of EUCRISA once daily over 52 weeks in pediatric (3 months to less than 18 years of age) and adult subjects with mild to moderate atopic dermatitis, who achieved success on EUCRISA twice daily during open-label treatment of up to 8 weeks.
A total of 497 subjects 3 months of age and older with a 2% to 90% treatable BSA, entered into an open-label period to receive EUCRISA twice daily for up to 8 weeks. At baseline, 327 (66%) of subjects were 3 months to less than 18 years of age, 66% of the subjects had an ISGA of moderate (3), and 34% had an ISGA of mild (2), in the overall assessment of atopic dermatitis (erythema, induration/papulation, and oozing/crusting) on a severity scale of 0 to 4.
Of the 497, a total of 254 subjects 3 months of age and older, who achieved both ISGA success (score of clear [0] or almost clear [1] with a ≥2 grade improvement from baseline) and EASI50 response (at least 50% improvement from baseline in EASI scores) were randomized 1:1 into a double-blind period to receive EUCRISA once daily or vehicle for 52 weeks or until they developed a flare. At the beginning of the double-blind period, 59% of the subjects had an ISGA of almost clear (1) and 41% had an ISGA of clear (0).
Figure 2 presents the percentage of subjects maintaining an ISGA of clear or almost clear through Week 52.
Figure 2: Percentage of Subjects Maintaining ISGA of Clear or Almost Clear Through Week 52