EMCYT®
(estramustine sodium phosphate)
Find EMCYT® medical information:
Find EMCYT® medical information:
EMCYT® Quick Finder
Indications and Usage
Dosage and Administration
DOSAGE AND ADMINISTRATION
The recommended daily dose is 14 mg per kg of body weight (ie, one 140 mg capsule for each 10 kg or 22 lb of body weight), given in 3 or 4 divided doses. Most patients in studies in the United States have been treated at a dosage range of 10 to 16 mg per kg per day.
Patients should be instructed to take EMCYT Capsules at least 1 hour before or 2 hours after meals. EMCYT should be swallowed with water. Milk, milk products, and calcium-rich foods or drugs (such as calcium-containing antacids) must not be taken simultaneously with EMCYT.
Patients should be treated for 30 to 90 days before the physician determines the possible benefits of continued therapy. Therapy should be continued as long as the favorable response lasts. Some patients have been maintained on therapy for more than 3 years at doses ranging from 10 to 16 mg per kg of body weight per day.
Procedures for proper handling and disposal of anticancer drugs should be considered. Several guidelines on this subject have been published.1–8 There is no general agreement that all of the procedures recommended in the guidelines are necessary or appropriate.
Contraindications
CONTRAINDICATIONS
EMCYT Capsules should not be used in patients with any of the following conditions:
- 1)
- Known hypersensitivity to either estradiol or to nitrogen mustard.
- 2)
- Active thrombophlebitis or thromboembolic disorders, except in those cases where the actual tumor mass is the cause of the thromboembolic phenomenon and the physician feels the benefits of therapy may outweigh the risks.
Warnings and Precautions
WARNINGS
It has been shown that there is an increased risk of thrombosis, including fatal and nonfatal myocardial infarction, in men receiving estrogens for prostatic cancer. EMCYT Capsules should be used with caution in patients with a history of thrombophlebitis, thrombosis, or thromboembolic disorders, especially if they were associated with estrogen therapy. Caution should also be used in patients with cerebral vascular or coronary artery disease.
Glucose Tolerance—Because glucose tolerance may be decreased, diabetic patients should be carefully observed while receiving EMCYT.
Elevated Blood Pressure—Because hypertension may occur, blood pressure should be monitored periodically.
PRECAUTIONS
General
Fluid Retention. Exacerbation of preexisting or incipient peripheral edema or congestive heart disease has been seen in some patients receiving therapy with EMCYT Capsules. Other conditions which might be influenced by fluid retention, such as epilepsy, migraine, or renal dysfunction, require careful observation.
EMCYT may be poorly metabolized in patients with impaired liver function and should be administered with caution in such patients.
Because EMCYT may influence the metabolism of calcium and phosphorus, it should be used with caution in patients with metabolic bone diseases that are associated with hypercalcemia or in patients with renal insufficiency. Patients with prostate cancer and osteoblastic metastases are at risk for hypocalcemia and should have calcium levels closely monitored.
Gynecomastia and impotence are known estrogenic effects.
Allergic reactions and angioedema at times involving the airway have been reported.
Information for the Patient
Because of the possibility of mutagenic effects, patients should be advised to use contraceptive measures.
Laboratory Tests
Certain endocrine and liver function tests may be affected by estrogen-containing drugs. EMCYT may depress testosterone levels. Abnormalities of hepatic enzymes and of bilirubin have occurred in patients receiving EMCYT. Such tests should be done at appropriate intervals during therapy and repeated after the drug has been withdrawn for two months.
Food/Drug Interaction
Milk, milk products, and calcium-rich foods or drugs may impair the absorption of EMCYT.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term continuous administration of estrogens in certain animal species increases the frequency of carcinomas of the breast and liver. Compounds structurally similar to EMCYT are carcinogenic in mice. Carcinogenic studies of EMCYT have not been conducted in man. Although testing by the Ames method failed to demonstrate mutagenicity for estramustine phosphate sodium, it is known that both estradiol and nitrogen mustard are mutagenic. For this reason and because some patients who had been impotent while on estrogen therapy have regained potency while taking EMCYT, the patient should be advised to use contraceptive measures.
Adverse Reactions
ADVERSE REACTIONS
In a randomized, double-blind trial comparing therapy with EMCYT Capsules in 93 patients (11.5 to 15.9 mg/kg/day) or diethylstilbestrol (DES) in 93 patients (3.0 mg/day), the following adverse effects were reported:
| EMCYT n=93 | DES n=93 | |
|---|---|---|
CARDIOVASCULAR-RESPIRATORY | ||
Cardiac Arrest | 0 | 2 |
Cerebrovascular Accident | 2 | 0 |
Myocardial Infarction | 3 | 1 |
Thrombophlebitis | 3 | 7 |
Pulmonary Emboli | 2 | 5 |
Congestive Heart Failure | 3 | 2 |
Edema | 19 | 17 |
Dyspnea | 11 | 3 |
Leg Cramps | 8 | 11 |
Upper Respiratory Discharge | 1 | 1 |
Hoarseness | 1 | 0 |
GASTROINTESTINAL | ||
Nausea | 15 | 8 |
Diarrhea | 12 | 11 |
Minor Gastrointestinal Upset | 11 | 6 |
Anorexia | 4 | 3 |
Flatulence | 2 | 0 |
Vomiting | 1 | 1 |
Gastrointestinal Bleeding | 1 | 0 |
Burning Throat | 1 | 0 |
Thirst | 1 | 0 |
INTEGUMENTARY | ||
Rash | 1 | 4 |
Pruritus | 2 | 2 |
Dry Skin | 2 | 0 |
Pigment Changes | 0 | 3 |
Easy Bruising | 3 | 0 |
Flushing | 1 | 0 |
Night Sweats | 0 | 1 |
Fingertip—Peeling Skin | 1 | 0 |
Thinning Hair | 1 | 1 |
BREAST CHANGES | ||
Tenderness | 66 | 64 |
Enlargement | ||
Mild | 60 | 54 |
Moderate | 10 | 16 |
Marked | 0 | 5 |
MISCELLANEOUS | ||
Lethargy Alone | 4 | 3 |
Depression | 0 | 2 |
Emotional Lability | 2 | 0 |
Insomnia | 3 | 0 |
Headache | 1 | 1 |
Anxiety | 1 | 0 |
Chest Pain | 1 | 1 |
Hot Flashes | 0 | 1 |
Pain in Eyes | 0 | 1 |
Tearing of Eyes | 1 | 1 |
Tinnitus | 0 | 1 |
LABORATORY ABNORMALITIES | ||
Hematologic | ||
Leukopenia | 4 | 2 |
Thrombopenia | 1 | 2 |
Hepatic | ||
Bilirubin Alone | 1 | 5 |
Bilirubin and LDH | 0 | 1 |
Bilirubin and SGOT | 2 | 1 |
Bilirubin, LDH and SGOT | 2 | 0 |
LDH and/or SGOT | 31 | 28 |
Miscellaneous | ||
Hypercalcemia—Transient | 0 | 1 |
Overdosage
OVERDOSAGE
Although there has been no experience with overdosage to date, it is reasonable to expect that such episodes may produce pronounced manifestations of the known adverse reactions. In the event of overdosage, the gastric contents should be evacuated by gastric lavage and symptomatic therapy should be initiated. Hematologic and hepatic parameters should be monitored for at least 6 weeks after overdosage of EMCYT Capsules.
Description
DESCRIPTION
Estramustine phosphate sodium, an antineoplastic agent, is an off-white powder readily soluble in water. EMCYT Capsules are white and opaque, each containing estramustine phosphate sodium as the disodium salt monohydrate equivalent to 140 mg estramustine phosphate, for oral administration. Each capsule also contains magnesium stearate, silicon dioxide, sodium lauryl sulfate, and talc. Gelatin capsule shells contain the following pigment: titanium dioxide.
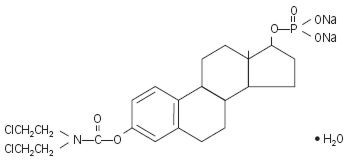
Chemically, estramustine phosphate sodium is estra-1,3,5(10)-triene-3,17-diol(17β)-,3-[bis(2-chloroethyl)carbamate] 17-(dihydrogen phosphate), disodium salt, monohydrate. It is also referred to as estradiol 3-[bis(2-chloroethyl)carbamate] 17-(dihydrogen phosphate), disodium salt, monohydrate.
Estramustine phosphate sodium has an empiric formula of C23H30Cl2NNa2O6P•H2O, a calculated molecular weight of 582.4, and the following structural formula:
Clinical Pharmacology
CLINICAL PHARMACOLOGY
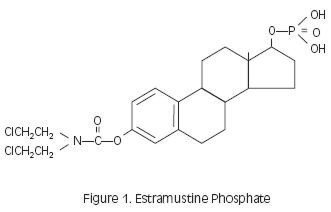
Estramustine phosphate (Figure 1) is a molecule combining estradiol and nornitrogen mustard by a carbamate link. The molecule is phosphorylated to make it water soluble.
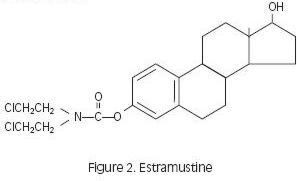
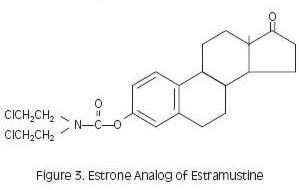
Estramustine phosphate taken orally is readily dephosphorylated during absorption, and the major metabolites in plasma are estramustine (Figure 2), the estrone analog (Figure 3), estradiol, and estrone.
Prolonged treatment with estramustine phosphate produces elevated total plasma concentrations of estradiol that fall within ranges similar to the elevated estradiol levels found in prostatic cancer patients given conventional estradiol therapy. Estrogenic effects, as demonstrated by changes in circulating levels of steroids and pituitary hormones, are similar in patients treated with either estramustine phosphate or conventional estradiol.
The metabolic urinary patterns of the estradiol moiety of estramustine phosphate and estradiol itself are very similar, although the metabolites derived from estramustine phosphate are excreted at a slower rate.
References
REFERENCES
- 1.
- Recommendations for the Safe Handling of Parenteral Antineoplastic Drugs. NIH Publication No. 83–2621. For sale by the Superintendent of Documents, U.S. Government Printing Office, Washington, DC, 20402.
- 2.
- AMA Council Report, Guidelines for Handling Parenteral Antineoplastics, JAMA. 1985; 253 (11):1590–1592.
- 3.
- National Study Commission on Cytotoxic Exposure-Recommendations for Handling Cytotoxic Agents. Available from Louis P. Jeffrey, Sc.D., Chairman, National Study Commission on Cytotoxic Exposure, Massachusetts College of Pharmacy and Allied Health Sciences, 179 Longwood Avenue, Boston, Massachusetts 02115.
- 4.
- Clinical Oncological Society of Australia. Guidelines and Recommendations for Safe Handling of Antineoplastic Agents. Med J Australia. 1983; 1:426–428.
- 5.
- Jones RB, et al. Safe Handling of Chemotherapeutic Agents: A Report from the Mount Sinai Medical Center. CA-A Cancer Journal for Clinicians. 1983; (Sept/Oct) 258–263.
- 6.
- American Society of Hospital Pharmacists Technical Assistance Bulletin on Handling Cytotoxic and Hazardous Drugs. Am J Hosp Pharm. 1990; 47:1033–1049.
- 7.
- OSHA Work-Practice Guidelines for Personnel Dealing with Cytotoxic (Antineoplastic) Drugs. Am J Hosp Pharm. 1986; 43:1193–1204.
- 8.
- ONS Clinical Practice Committee. Cancer Chemotherapy Guidelines and Recommendations for Practice. Pittsburgh, Pa: Oncology Nursing Society; 1999:32–41.
This product’s label may have been updated. For current full prescribing information, please visit www.pfizer.com. For medical information about EMCYT, please visit www.pfizermedinfo.com or call 1‑800-438-1985.
Rx only
LAB-0088-5.0
Revised April 2023
How Supplied/Storage and Handling
Resources
Didn’t find what you were looking for?
Contact us.
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine.
*Speak with a Pfizer Medical Information Professional regarding your medical inquiry. Available 9AM-5Pm ET Monday to Friday; excluding holidays.
Submit a medical question for Pfizer prescription products.
Report Adverse Event
To report an adverse event related to a Pfizer product and you are not part of a clinical trial* for this product, click the link below to submit your information: Pfizer Safety Reporting Site
*If you are involved in a clinical trial for either product, adverse events should be reported to your coordinating study site.
If you cannot use the above website to report an adverse event related to a Pfizer product, please call Pfizer Medical Information at (800) 438-1985.
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or by calling (800)-332-1088.