DESCRIPTION
25% Dextrose Injection, USP is a sterile, nonpyrogenic, hypertonic solution of dextrose in water for injection administered by intravenous injection to restore blood glucose levels in hypoglycemia and as a source of carbohydrate calories. Each milliliter (mL) of fluid contains dextrose, hydrous, 250 mg which delivers 3.4 kcal/gram (0.85 kcal/mL). The solution has an osmolarity of 1.39 mOsmol/mL (calc.). pH is 3.2 to 6.5. May contain hydrochloric acid and sodium hydroxide for pH adjustment.
The solution contains no bacteriostat, antimicrobial agent or added buffer (except for pH adjustment) and is intended only for use as a single-dose injection. When smaller doses are required the unused portion should be discarded with the entire unit.
25% Dextrose Injection, USP is a dextrose (glucose) and nutrient (carbohydrate) replenisher.
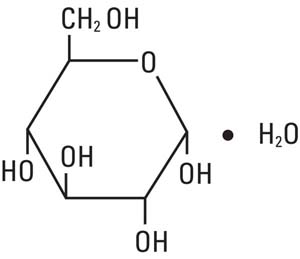
Dextrose, USP is chemically designated D-glucose monohydrate, (C6H12O6 • H2O), a hexose sugar freely soluble in water.
It has the following structural formula:
The syringe is molded from a specially formulated polypropylene. Water permeates from inside the container at an extremely slow rate which will have an insignificant effect on solution concentration over the expected shelf life. Solutions in contact with the plastic container may leach out certain chemical components from the plastic in very small amounts; however, biological testing was supportive of the safety of the syringe material.