Dextrose 5% ADD-Vantage™ Diluent How Supplied/Storage and Handling
()
HOW SUPPLIED
5% Dextrose Injection, USP is supplied in single-dose flexible plastic ADD-VantageTM diluent containers.
Store at 20 to 25°C (68 to 77°F). [See USP Controlled Room Temperature.]
| Unit of Sale | Volume |
|---|---|
| NDC 0409-7100-66 Case of 50 – 50 mL bags | 50 mL bags |
| NDC 0409-7100-67 Case of 50 – 100 mL bags | 100 mL bags |
| NDC 0409-7100-02 Case of 24 – 250 mL bags | 250 mL bags |
INSTRUCTIONS FOR USE WITH ADD-VANTAGE VIAL
These instructions for use should be made available to the individuals who perform the reconstitution steps.
To Open:
Peel overwrap at corner and remove solution container. Use unit within 30 days of opening overwrap, as long as the use date does not exceed the printed expiration date. Some opacity of the plastic due to moisture absorption during the sterilization process may be observed. This is normal and does not affect the solution quality or safety. The opacity will diminish gradually.
To Assemble Vial and Flexible Diluent Container:
(Use Aseptic Technique)
- 1.
- Remove the protective covers from the top of the vial and the vial port on the diluent container as follows:
- a.
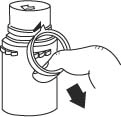
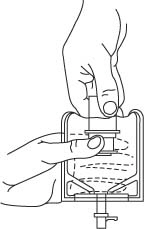
- To remove the breakaway vial cap, swing the pull ring over the top of the vial and pull down far enough to start the opening (SEE FIGURE 1.), then pull straight up to remove the cap. (SEE FIGURE 2.)
NOTE: Once the breakaway cap has been removed, do not access vial with syringe.
- b.
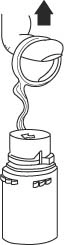
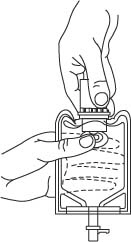
- To remove the vial port cover, grasp the tab on the pull ring, pull up to break the three tie strings, then pull back to remove the cover. (SEE FIGURE 3.)
- 2.
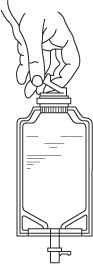
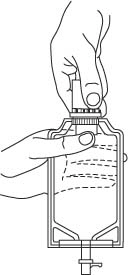
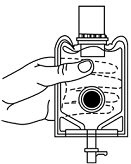
- Screw the vial into the vial port until it will go no further. THE VIAL MUST BE SCREWED IN TIGHTLY TO ASSURE A SEAL. This occurs approximately 1/2 turn (180°) after the first audible click. (SEE FIGURE 4.) The clicking sound does not assure a seal; the vial must be turned as far as it will go.
NOTE: Once vial is seated, do not attempt to remove. (SEE FIGURE 4.) - 3.
- Recheck the vial to assure that it is tight by trying to turn it further in the direction of assembly.
- 4.
- Label appropriately.
Fig. 3 Fig. 4
Preparation for Administration:
(Use Aseptic Technique)
- 1.
- Confirm the activation and admixture of vial contents.
- 2.
- Check for leaks by squeezing container firmly. If leaks are found, discard unit as sterility may be impaired.
- 3.
- Close flow control clamp of administration set.
- 4.
- Remove cover from outlet port at bottom of container.
- 5.
- Insert piercing pin of administration set into port with a twisting motion until the pin is firmly seated. NOTE: See full directions on administration set carton.
- 6.
- Lift the free end of the hanger loop on the bottom of the vial, breaking the two tie strings. Bend the loop outward to lock it in the upright position, then suspend container from hanger.
- 7.
- Squeeze and release drip chamber to establish proper fluid level in chamber.
- 8.
- Open flow control clamp and clear air from set. Close clamp.
- 9.
- Attach set to venipuncture device. If device is not indwelling, prime and make venipuncture.
- 10.
- Regulate rate of administration with flow control clamp.
WARNING: Do not use flexible container in series connections.
INSTRUCTIONS FOR USE WITH ADD-VANTAGE ADDAPTORTM
The instructions for use provided with the ADD-Vantage ADDAPTORTM should be made available to the individuals who perform the reconstitution steps.
Distributed by Hospira, Inc., Lake Forest, IL 60045 USA 
LAB-1143-3.0
Revised: 09/2021
Find Dextrose 5% ADD-Vantage™ Diluent medical information:
Find Dextrose 5% ADD-Vantage™ Diluent medical information:
Dextrose 5% ADD-Vantage™ Diluent Quick Finder
Health Professional Information
How Supplied/Storage and Handling
HOW SUPPLIED
5% Dextrose Injection, USP is supplied in single-dose flexible plastic ADD-VantageTM diluent containers.
Store at 20 to 25°C (68 to 77°F). [See USP Controlled Room Temperature.]
| Unit of Sale | Volume |
|---|---|
| NDC 0409-7100-66 Case of 50 – 50 mL bags | 50 mL bags |
| NDC 0409-7100-67 Case of 50 – 100 mL bags | 100 mL bags |
| NDC 0409-7100-02 Case of 24 – 250 mL bags | 250 mL bags |
INSTRUCTIONS FOR USE WITH ADD-VANTAGE VIAL
These instructions for use should be made available to the individuals who perform the reconstitution steps.
To Open:
Peel overwrap at corner and remove solution container. Use unit within 30 days of opening overwrap, as long as the use date does not exceed the printed expiration date. Some opacity of the plastic due to moisture absorption during the sterilization process may be observed. This is normal and does not affect the solution quality or safety. The opacity will diminish gradually.
To Assemble Vial and Flexible Diluent Container:
(Use Aseptic Technique)
- 1.
- Remove the protective covers from the top of the vial and the vial port on the diluent container as follows:
- a.
- To remove the breakaway vial cap, swing the pull ring over the top of the vial and pull down far enough to start the opening (SEE FIGURE 1.), then pull straight up to remove the cap. (SEE FIGURE 2.)
NOTE: Once the breakaway cap has been removed, do not access vial with syringe.
- b.
- To remove the vial port cover, grasp the tab on the pull ring, pull up to break the three tie strings, then pull back to remove the cover. (SEE FIGURE 3.)
- 2.
- Screw the vial into the vial port until it will go no further. THE VIAL MUST BE SCREWED IN TIGHTLY TO ASSURE A SEAL. This occurs approximately 1/2 turn (180°) after the first audible click. (SEE FIGURE 4.) The clicking sound does not assure a seal; the vial must be turned as far as it will go.
NOTE: Once vial is seated, do not attempt to remove. (SEE FIGURE 4.) - 3.
- Recheck the vial to assure that it is tight by trying to turn it further in the direction of assembly.
- 4.
- Label appropriately.
Fig. 3 Fig. 4
Preparation for Administration:
(Use Aseptic Technique)
- 1.
- Confirm the activation and admixture of vial contents.
- 2.
- Check for leaks by squeezing container firmly. If leaks are found, discard unit as sterility may be impaired.
- 3.
- Close flow control clamp of administration set.
- 4.
- Remove cover from outlet port at bottom of container.
- 5.
- Insert piercing pin of administration set into port with a twisting motion until the pin is firmly seated. NOTE: See full directions on administration set carton.
- 6.
- Lift the free end of the hanger loop on the bottom of the vial, breaking the two tie strings. Bend the loop outward to lock it in the upright position, then suspend container from hanger.
- 7.
- Squeeze and release drip chamber to establish proper fluid level in chamber.
- 8.
- Open flow control clamp and clear air from set. Close clamp.
- 9.
- Attach set to venipuncture device. If device is not indwelling, prime and make venipuncture.
- 10.
- Regulate rate of administration with flow control clamp.
WARNING: Do not use flexible container in series connections.
INSTRUCTIONS FOR USE WITH ADD-VANTAGE ADDAPTORTM
The instructions for use provided with the ADD-Vantage ADDAPTORTM should be made available to the individuals who perform the reconstitution steps.
Distributed by Hospira, Inc., Lake Forest, IL 60045 USA 
LAB-1143-3.0
Revised: 09/2021
Resources
Didn’t find what you were looking for? Contact us.
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine.
*Speak with a Pfizer Medical Information Professional regarding your medical inquiry. Available 9AM-5Pm ET Monday to Friday; excluding holidays.
Submit a medical question for Pfizer prescription products.
Report Adverse Event
To report an adverse event related to the Pfizer-BioNTech COVID-19 Vaccine, and you are not part of a clinical trial* for this product, click the link below to submit your information:
Pfizer Safety Reporting Site*If you are involved in a clinical trial for this product, adverse events should be reported to your coordinating study site.
If you cannot use the above website, or would like to report an adverse event related to a different Pfizer product, please call Pfizer Safety at (800) 438-1985.
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or call (800) 822-7967.