DEPO-SUBQ PROVERA 104® Warnings and Precautions
(medroxyprogesterone acetate)
5 WARNINGS AND PRECAUTIONS
5.1 Loss of Bone Mineral Density
Use of depo-subQ provera 104 reduces serum estrogen levels and is associated with significant loss of bone mineral density (BMD). This loss of BMD is of particular concern during adolescence and early adulthood, a critical period of bone accretion. It is unknown if use of depo-subQ provera 104 by younger women will reduce peak bone mass and increase the risk for osteoporotic fracture in later life.
A study to assess the reversibility of loss of BMD in adolescents was conducted with DMPA-IM. After discontinuing DMPA-IM in these adolescents, mean BMD loss at the total hip and femoral neck did not fully recover by 5 years (60 months) post-treatment in the sub-group of adolescents who were treated for more than 2 years [see Clinical Studies (14.4)]. Similarly, in adults, there was only partial recovery of mean BMD at the total hip, femoral neck, and lumbar spine towards baseline by 2 years post-treatment [see Clinical Studies (14.3)].
The use of depo-subQ provera 104 is not recommended as a long-term (i.e., longer than 2 years) birth control method or medical therapy for endometriosis-associated pain unless other options are considered inadequate. BMD should be evaluated when a woman needs to continue to use depo-subQ provera 104 long-term. In adolescents, interpretation of BMD results should take into account patient age and skeletal maturity.
Other birth control methods or therapies for endometriosis-associated pain should be considered in the risk/benefit analysis for the use of depo-subQ provera 104 in women with osteoporosis risk factors. Depo-subQ provera 104 can pose an additional risk in patients with risk factors for osteoporosis (e.g., metabolic bone disease, chronic alcohol and/or tobacco use, anorexia nervosa, strong family history of osteoporosis, or chronic use of drugs that can reduce bone mass such as anticonvulsants or corticosteroids).
5.2 Arterial and Venous Thromboembolic Disorders
There have been reports of serious arterial and venous thrombotic events in women treated with DMPA-IM. Women with a history of thromboembolic disorders were not studied in clinical trials of depo-subQ provera 104. Although no causal relationship between the use of depo-subQ provera 104 and thrombotic events has been clearly established, patients who develop arterial or venous thrombosis while taking depo-subQ provera 104 should discontinue treatment.
Do not re-administer depo-subQ provera 104 pending examination if there is a sudden onset of a suspected vascular ocular event (e.g., partial or complete loss of vision, proptosis, or diplopia) or migraine. Do not re-administer depo-subQ provera 104 if examination reveals papilledema or retinal vascular lesions.
5.3 Cancer Risks
Breast Cancer
The use of hormonal contraceptives, including depo sub-Q provera 104, is contraindicated in women who have or have had breast cancer because breast cancer may be sensitive to hormones [see Contraindications (4)]. Women who have a family history of breast cancer or a significant risk of breast cancer should be monitored.
The results of five large case-control studies assessing the association between DMPA-IM use and the risk of breast cancer are summarized in Figure M. Three of the studies suggest a slightly increased risk of breast cancer in the overall population of users; these increased risks were statistically significant in one study. One US study1 evaluated the timing and duration of use and found a statistically significant increased risk of breast cancer in recent DMPA-IM users (defined as last use within the past five years) who used DMPA-IM for 12 months or longer; this is consistent with results of a previous study2.
| Figure M. Risk Estimates of Breast Cancer in DMPA-IM Users |
|---|
| Odds ratio estimates were adjusted for the following covariates: Lee et al. (1987): age, parity, and socioeconomic status. Paul et al. (1989): age, parity, ethnic group, and year of interview. WHO (1991): age, center, and age at first live birth. Shapiro et al. (2000): age, ethnic group, socioeconomic status, and any combined estrogen/progestogen oral contraceptive use. Li et al. (2012): age, year, BMI, duration of OC use, number of full-term pregnancies, family history of breast cancer, and history of screening mammography. |
| Odds Ratio [95% confidence interval (CI)] displayed on logarithmic scale |
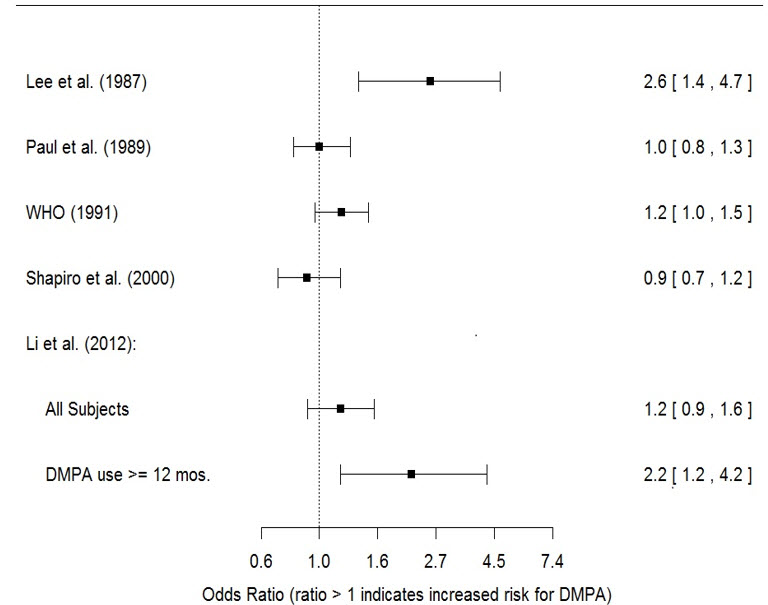 |
Based on the published SEER-18 2015 incidence rate (age-adjusted to the 2000 US Standard Population) of breast cancer for US women, all races, age 20 to 49 years, a doubling of risk would increase the incidence of breast cancer in women who use DMPA-IM from about 73 to about 146 cases per 100,000 women.
Other Cancers
The relative rate of invasive squamous-cell cervical cancer in women who ever used DMPA-IM was estimated to be 1.11 (95% CI: 0.96 to 1.29). No trends in risk with duration of use or times since initial or most recent exposure were observed.
Long-term, case-controlled surveillance of users of DMPA-IM found no overall increased risk of ovarian or liver cancer.
5.4 Ectopic Pregnancy
Healthcare professionals should be alert to the possibility of an ectopic pregnancy among women using depo-subQ provera 104 who become pregnant or complain of severe abdominal pain.
5.5 Anaphylaxis
Serious anaphylactic reactions have been reported in women using depo-subQ provera 104. If an anaphylactic reaction occurs, appropriate emergency medical treatment should be administered.
5.6 Fluid Retention
Because progestational drugs including depo-subQ provera 104 may cause fluid retention, monitor patients with conditions that might be affected by fluid retention.
5.7 Weight Gain
Weight gain is a common occurrence in women using depo-subQ provera 104. In three large clinical trials using depo-subQ provera 104, the mean weight gain was 3.5 lb (1.6 kg) in the first year of use. In a small, two-year study comparing depo-subQ provera 104 to DMPA-IM, the mean weight gain observed for women using depo-subQ provera 104 [7.5 lb (3.4 kg)] was similar to the mean weight gain for women using DMPA-IM [7.6 lb (3.5 kg)].
Although there are no data related to weight gain beyond 2 years for depo-subQ provera 104, the data on DMPA-IM may be relevant. In a clinical study, after five years, 41 women using Depo-Provera CI (150 mg) had a mean weight gain of 11.2 lb (5.1 kg), while 114 women using non-hormonal contraception had a mean weight gain of 6.4 lb (2.9 kg).
5.8 Delayed Return of Ovulation or Fertility
Return to ovulation is likely to be delayed after stopping depo-subQ provera 104, as demonstrated in a study of 15 women who received multiple doses of depo-subQ provera 104:
- Median time to ovulation was 10 months after the last injection.
- Earliest return to ovulation was 6 months after the last injection.
- 12 women (80%) ovulated within 1 year of the last injection.
However, ovulation has occurred as early as 14 weeks after a single dose of depo-subQ provera 104; therefore, administer the next depo-subQ provera 104 12 to 14 weeks after the last injection.
Return to fertility also is likely to be delayed after stopping therapy. Among 28 women using depo-subQ provera 104 for contraception who stopped treatment to become pregnant, 7 women were lost to follow-up. One woman became pregnant within one year of her last injection and another woman became pregnant 443 days after her last injection. The remaining 19 women had not become pregnant; it is not known if these 19 women were still attempting to become pregnant or if they had started a new contraceptive method.
5.9 Depression
Depression (3% of depo-subQ provera 104-treated patients) and other mood disorders have been reported in clinical trials of depo-subQ provera 104 [see Adverse Reactions (6.1)]. Patients with a history of depression or who are on treatment for depression may be at increased risk for depression recurrence or exacerbation and for associated mood disorders while receiving depo-subQ provera 104. Therefore, patients should be monitored for symptoms of depression and mood changes.
5.10 Injection Site Reactions
In five clinical studies of depo-subQ provera 104 involving 2325 women (282 treated for up to 6 months, 1780 treated for up to 1 year, and 263 women treated for up to 2 years), 5% of women reported injection site reactions, and 1% had persistent skin changes (small areas of induration or atrophy).
In post-marketing experience, injection site reactions such as persistent atrophy of the injection site, dimpling/indentation, and injection site lump/nodule have been reported.
5.11 Bleeding Irregularities
Most women using depo-subQ provera 104 experienced changes in menstrual bleeding patterns, such as amenorrhea, irregular unpredictable spotting or bleeding, prolonged spotting or bleeding, or heavy bleeding [see Adverse Reactions (6.1)]. Fewer women experienced irregular bleeding and more experienced amenorrhea with longer term use of depo-subQ provera 104, consistent with expected endometrial thinning effects.
In three contraception trials, 39% of 2053 depo-subQ provera 104-treated women experienced amenorrhea during Month 6, and 57% experienced amenorrhea during Month 12. In two endometriosis trials using depo-subQ provera 104, 24% of 289 women experienced amenorrhea during Month 6 [see Adverse Reactions (6.1)].
If abnormal bleeding is persistent or severe, evaluate the patient for underlying pathology or pregnancy.
5.12 Risk of Hyperglycemia in Patients with Diabetes
Some patients receiving progestins may exhibit a decrease in glucose tolerance; therefore, patients with diabetes may be at greater risk of hyperglycemia.
5.13 Jaundice and Elevated Transaminase
Discontinue depo-subQ provera 104 if jaundice or elevated transaminase levels develop. Depo-subQ provera 104 may be resumed after both the jaundice and elevated transaminase levels resolve, and the healthcare professional determines that depo-subQ provera 104 did not cause the abnormalities.
Find DEPO-SUBQ PROVERA 104® medical information:
Find DEPO-SUBQ PROVERA 104® medical information:
DEPO-SUBQ PROVERA 104® Quick Finder
Health Professional Information
Warnings and Precautions
5 WARNINGS AND PRECAUTIONS
5.1 Loss of Bone Mineral Density
Use of depo-subQ provera 104 reduces serum estrogen levels and is associated with significant loss of bone mineral density (BMD). This loss of BMD is of particular concern during adolescence and early adulthood, a critical period of bone accretion. It is unknown if use of depo-subQ provera 104 by younger women will reduce peak bone mass and increase the risk for osteoporotic fracture in later life.
A study to assess the reversibility of loss of BMD in adolescents was conducted with DMPA-IM. After discontinuing DMPA-IM in these adolescents, mean BMD loss at the total hip and femoral neck did not fully recover by 5 years (60 months) post-treatment in the sub-group of adolescents who were treated for more than 2 years [see Clinical Studies (14.4)]. Similarly, in adults, there was only partial recovery of mean BMD at the total hip, femoral neck, and lumbar spine towards baseline by 2 years post-treatment [see Clinical Studies (14.3)].
The use of depo-subQ provera 104 is not recommended as a long-term (i.e., longer than 2 years) birth control method or medical therapy for endometriosis-associated pain unless other options are considered inadequate. BMD should be evaluated when a woman needs to continue to use depo-subQ provera 104 long-term. In adolescents, interpretation of BMD results should take into account patient age and skeletal maturity.
Other birth control methods or therapies for endometriosis-associated pain should be considered in the risk/benefit analysis for the use of depo-subQ provera 104 in women with osteoporosis risk factors. Depo-subQ provera 104 can pose an additional risk in patients with risk factors for osteoporosis (e.g., metabolic bone disease, chronic alcohol and/or tobacco use, anorexia nervosa, strong family history of osteoporosis, or chronic use of drugs that can reduce bone mass such as anticonvulsants or corticosteroids).
5.2 Arterial and Venous Thromboembolic Disorders
There have been reports of serious arterial and venous thrombotic events in women treated with DMPA-IM. Women with a history of thromboembolic disorders were not studied in clinical trials of depo-subQ provera 104. Although no causal relationship between the use of depo-subQ provera 104 and thrombotic events has been clearly established, patients who develop arterial or venous thrombosis while taking depo-subQ provera 104 should discontinue treatment.
Do not re-administer depo-subQ provera 104 pending examination if there is a sudden onset of a suspected vascular ocular event (e.g., partial or complete loss of vision, proptosis, or diplopia) or migraine. Do not re-administer depo-subQ provera 104 if examination reveals papilledema or retinal vascular lesions.
5.3 Cancer Risks
Breast Cancer
The use of hormonal contraceptives, including depo sub-Q provera 104, is contraindicated in women who have or have had breast cancer because breast cancer may be sensitive to hormones [see Contraindications (4)]. Women who have a family history of breast cancer or a significant risk of breast cancer should be monitored.
The results of five large case-control studies assessing the association between DMPA-IM use and the risk of breast cancer are summarized in Figure M. Three of the studies suggest a slightly increased risk of breast cancer in the overall population of users; these increased risks were statistically significant in one study. One US study1 evaluated the timing and duration of use and found a statistically significant increased risk of breast cancer in recent DMPA-IM users (defined as last use within the past five years) who used DMPA-IM for 12 months or longer; this is consistent with results of a previous study2.
| Figure M. Risk Estimates of Breast Cancer in DMPA-IM Users |
|---|
| Odds ratio estimates were adjusted for the following covariates: Lee et al. (1987): age, parity, and socioeconomic status. Paul et al. (1989): age, parity, ethnic group, and year of interview. WHO (1991): age, center, and age at first live birth. Shapiro et al. (2000): age, ethnic group, socioeconomic status, and any combined estrogen/progestogen oral contraceptive use. Li et al. (2012): age, year, BMI, duration of OC use, number of full-term pregnancies, family history of breast cancer, and history of screening mammography. |
| Odds Ratio [95% confidence interval (CI)] displayed on logarithmic scale |
 |
Based on the published SEER-18 2015 incidence rate (age-adjusted to the 2000 US Standard Population) of breast cancer for US women, all races, age 20 to 49 years, a doubling of risk would increase the incidence of breast cancer in women who use DMPA-IM from about 73 to about 146 cases per 100,000 women.
Other Cancers
The relative rate of invasive squamous-cell cervical cancer in women who ever used DMPA-IM was estimated to be 1.11 (95% CI: 0.96 to 1.29). No trends in risk with duration of use or times since initial or most recent exposure were observed.
Long-term, case-controlled surveillance of users of DMPA-IM found no overall increased risk of ovarian or liver cancer.
5.4 Ectopic Pregnancy
Healthcare professionals should be alert to the possibility of an ectopic pregnancy among women using depo-subQ provera 104 who become pregnant or complain of severe abdominal pain.
5.5 Anaphylaxis
Serious anaphylactic reactions have been reported in women using depo-subQ provera 104. If an anaphylactic reaction occurs, appropriate emergency medical treatment should be administered.
5.6 Fluid Retention
Because progestational drugs including depo-subQ provera 104 may cause fluid retention, monitor patients with conditions that might be affected by fluid retention.
5.7 Weight Gain
Weight gain is a common occurrence in women using depo-subQ provera 104. In three large clinical trials using depo-subQ provera 104, the mean weight gain was 3.5 lb (1.6 kg) in the first year of use. In a small, two-year study comparing depo-subQ provera 104 to DMPA-IM, the mean weight gain observed for women using depo-subQ provera 104 [7.5 lb (3.4 kg)] was similar to the mean weight gain for women using DMPA-IM [7.6 lb (3.5 kg)].
Although there are no data related to weight gain beyond 2 years for depo-subQ provera 104, the data on DMPA-IM may be relevant. In a clinical study, after five years, 41 women using Depo-Provera CI (150 mg) had a mean weight gain of 11.2 lb (5.1 kg), while 114 women using non-hormonal contraception had a mean weight gain of 6.4 lb (2.9 kg).
5.8 Delayed Return of Ovulation or Fertility
Return to ovulation is likely to be delayed after stopping depo-subQ provera 104, as demonstrated in a study of 15 women who received multiple doses of depo-subQ provera 104:
- Median time to ovulation was 10 months after the last injection.
- Earliest return to ovulation was 6 months after the last injection.
- 12 women (80%) ovulated within 1 year of the last injection.
However, ovulation has occurred as early as 14 weeks after a single dose of depo-subQ provera 104; therefore, administer the next depo-subQ provera 104 12 to 14 weeks after the last injection.
Return to fertility also is likely to be delayed after stopping therapy. Among 28 women using depo-subQ provera 104 for contraception who stopped treatment to become pregnant, 7 women were lost to follow-up. One woman became pregnant within one year of her last injection and another woman became pregnant 443 days after her last injection. The remaining 19 women had not become pregnant; it is not known if these 19 women were still attempting to become pregnant or if they had started a new contraceptive method.
5.9 Depression
Depression (3% of depo-subQ provera 104-treated patients) and other mood disorders have been reported in clinical trials of depo-subQ provera 104 [see Adverse Reactions (6.1)]. Patients with a history of depression or who are on treatment for depression may be at increased risk for depression recurrence or exacerbation and for associated mood disorders while receiving depo-subQ provera 104. Therefore, patients should be monitored for symptoms of depression and mood changes.
5.10 Injection Site Reactions
In five clinical studies of depo-subQ provera 104 involving 2325 women (282 treated for up to 6 months, 1780 treated for up to 1 year, and 263 women treated for up to 2 years), 5% of women reported injection site reactions, and 1% had persistent skin changes (small areas of induration or atrophy).
In post-marketing experience, injection site reactions such as persistent atrophy of the injection site, dimpling/indentation, and injection site lump/nodule have been reported.
5.11 Bleeding Irregularities
Most women using depo-subQ provera 104 experienced changes in menstrual bleeding patterns, such as amenorrhea, irregular unpredictable spotting or bleeding, prolonged spotting or bleeding, or heavy bleeding [see Adverse Reactions (6.1)]. Fewer women experienced irregular bleeding and more experienced amenorrhea with longer term use of depo-subQ provera 104, consistent with expected endometrial thinning effects.
In three contraception trials, 39% of 2053 depo-subQ provera 104-treated women experienced amenorrhea during Month 6, and 57% experienced amenorrhea during Month 12. In two endometriosis trials using depo-subQ provera 104, 24% of 289 women experienced amenorrhea during Month 6 [see Adverse Reactions (6.1)].
If abnormal bleeding is persistent or severe, evaluate the patient for underlying pathology or pregnancy.
5.12 Risk of Hyperglycemia in Patients with Diabetes
Some patients receiving progestins may exhibit a decrease in glucose tolerance; therefore, patients with diabetes may be at greater risk of hyperglycemia.
5.13 Jaundice and Elevated Transaminase
Discontinue depo-subQ provera 104 if jaundice or elevated transaminase levels develop. Depo-subQ provera 104 may be resumed after both the jaundice and elevated transaminase levels resolve, and the healthcare professional determines that depo-subQ provera 104 did not cause the abnormalities.
Health Professional Information
{{section_name_patient}}
{{section_body_html_patient}}
Resources
Didn’t find what you were looking for? Contact us.
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine.
*Speak with a Pfizer Medical Information Professional regarding your medical inquiry. Available 9AM-5Pm ET Monday to Friday; excluding holidays.
Submit a medical question for Pfizer prescription products.
Report Adverse Event
To report an adverse event related to the Pfizer-BioNTech COVID-19 Vaccine, and you are not part of a clinical trial* for this product, click the link below to submit your information:
Pfizer Safety Reporting Site*If you are involved in a clinical trial for this product, adverse events should be reported to your coordinating study site.
If you cannot use the above website, or would like to report an adverse event related to a different Pfizer product, please call Pfizer Safety at (800) 438-1985.
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or call (800) 822-7967.