DEPO-PROVERA® CI Warnings and Precautions
(medroxyprogesterone acetate injectable suspension, for intramuscular use)
5 WARNINGS AND PRECAUTIONS
5.1 Loss of Bone Mineral Density
Use of Depo-Provera CI reduces serum estrogen levels and is associated with significant loss of bone mineral density (BMD). This loss of BMD is of particular concern during adolescence and early adulthood, a critical period of bone accretion. It is unknown if use of Depo-Provera CI by younger women will reduce peak bone mass and increase the risk for osteoporotic fracture in later life.
A study to assess the reversibility of loss of BMD in adolescents was conducted with Depo-Provera CI. After discontinuing Depo-Provera CI in these adolescents, mean BMD loss at the total hip and femoral neck did not fully recover by 5 years (60 months) post-treatment in the sub-group of adolescents who were treated for more than 2 years [see Clinical Studies (14.3)]. Similarly, in adults, there was only partial recovery of mean BMD at the total hip, femoral neck, and lumbar spine towards baseline by 2 years post-treatment [see Clinical Studies (14.2)].
The use of Depo-Provera CI is not recommended as a long-term (i.e., longer than 2 years) birth control method unless other options are considered inadequate. BMD should be evaluated when a woman needs to continue to use Depo-Provera CI long-term. In adolescents, interpretation of BMD results should take into account patient age and skeletal maturity.
Other birth control methods should be considered in the risk/benefit analysis for the use of Depo-Provera CI in women with osteoporosis risk factors. Depo-Provera CI can pose an additional risk in patients with risk factors for osteoporosis (e.g., metabolic bone disease, chronic alcohol and/or tobacco use, anorexia nervosa, strong family history of osteoporosis or chronic use of drugs that can reduce bone mass such as anticonvulsants or corticosteroids).
5.2 Thromboembolic Disorders
There have been reports of serious thrombotic events in women using Depo-Provera CI (150 mg). However, Depo-Provera CI has not been causally associated with the induction of thrombotic or thromboembolic disorders. Any patient who develops thrombosis while undergoing therapy with Depo-Provera CI should discontinue treatment unless she has no other acceptable options for birth control.
Do not re-administer Depo-Provera CI pending examination if there is a sudden partial or complete loss of vision or if there is a sudden onset of proptosis, diplopia, or migraine. Do not re-administer if examination reveals papilledema or retinal vascular lesions.
5.3 Cancer Risks
Breast Cancer
Women who have or have had a history of breast cancer should not use hormonal contraceptives, including Depo-Provera CI, because breast cancer may be hormonally sensitive [see Contraindications (4)]. Women with a strong family history of breast cancer should be monitored with particular care.
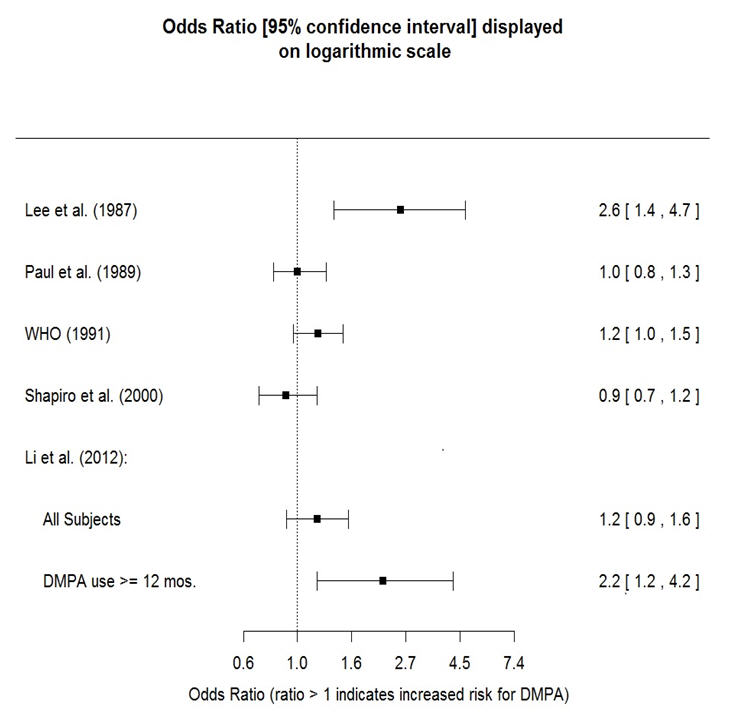
The results of five large case-control studies assessing the association between depo-medroxyprogesterone acetate (DMPA) use and the risk of breast cancer are summarized in Figure 1. Three of the studies suggest a slightly increased risk of breast cancer in the overall population of users; these increased risks were statistically significant in one study. One recent US study1 evaluated the recency and duration of use and found a statistically significantly increased risk of breast cancer in recent users (defined as last use within the past five years) who used DMPA for 12 months or longer; this is consistent with results of a previous study2.
Figure 1 Risk estimates for breast cancer in DMPA users
| Odds ratio estimates were adjusted for the following covariates: |
| Lee et al. (1987): age, parity, and socioeconomic status. |
| Paul et al. (1989): age, parity, ethnic group, and year of interview. |
| WHO (1991): age, center, and age at first live birth. |
| Shapiro et al. (2000): age, ethnic group, socioeconomic status, and any combined estrogen/progestogen oral contraceptive use. |
| Li et al. (2012): age, year, BMI, duration of OC use, number of full-term pregnancies, family history of breast cancer, and history of screening mammography. |
|
Based on the published SEER-18 2011 incidence rate (age-adjusted to the 2000 US Standard Population) of breast cancer for US women, all races, age 20 to 49 years, a doubling of risk would increase the incidence of breast cancer in women who use Depo-Provera CI from about 72 to about 144 cases per 100,000 women.
Cervical Cancer
A statistically nonsignificant increase in RR estimates of invasive squamous-cell cervical cancer has been associated with the use of Depo-Provera CI in women who were first exposed before the age of 35 years (RR 1.22 to 1.28 and 95% CI 0.93 to 1.70). The overall, nonsignificant relative rate of invasive squamous-cell cervical cancer in women who ever used Depo-Provera CI was estimated to be 1.11 (95% CI 0.96 to 1.29). No trends in risk with duration of use or times since initial or most recent exposure were observed.
5.4 Ectopic Pregnancy
Be alert to the possibility of an ectopic pregnancy among women using Depo-Provera CI who become pregnant or complain of severe abdominal pain.
5.5 Anaphylaxis and Anaphylactoid Reaction
Anaphylaxis and anaphylactoid reaction have been reported with the use of Depo-Provera CI. Institute emergency medical treatment if an anaphylactic reaction occurs.
5.6 Injection Site Reactions
Injection site reactions have been reported with use of Depo-Provera CI [see Adverse Reactions (6.2)]. Persistent injection site reactions may occur after administration of Depo-Provera CI due to inadvertent subcutaneous administration or release of the drug into the subcutaneous space while removing the needle [see Dosage and Administration (2.1)].
5.7 Liver Function
Discontinue Depo-Provera CI use if jaundice or acute or chronic disturbances of liver function develop. Do not resume use until markers of liver function return to normal and Depo-Provera CI causation has been excluded.
5.8 Convulsions
There have been a few reported cases of convulsions in patients who were treated with Depo-Provera CI. Association with drug use or pre-existing conditions is not clear.
5.9 Depression
Monitor patients who have a history of depression and do not re-administer Depo-Provera CI if depression recurs.
5.10 Bleeding Irregularities
Most women using Depo-Provera CI experience disruption of menstrual bleeding patterns. Altered menstrual bleeding patterns include amenorrhea, irregular or unpredictable bleeding or spotting, prolonged spotting or bleeding, and heavy bleeding. Rule out the possibility of organic pathology if abnormal bleeding persists or is severe, and institute appropriate treatment.
As women continue using Depo-Provera CI, fewer experience irregular bleeding and more experience amenorrhea. In clinical studies of Depo-Provera CI, by month 12 amenorrhea was reported by 55% of women, and by month 24, amenorrhea was reported by 68% of women using Depo-Provera CI.
5.11 Weight Gain
Women tend to gain weight while on therapy with Depo-Provera CI. From an initial average body weight of 136 lb, women who completed 1 year of therapy with Depo-Provera CI gained an average of 5.4 lb. Women who completed 2 years of therapy gained an average of 8.1 lb. Women who completed 4 years gained an average of 13.8 lb. Women who completed 6 years gained an average of 16.5 lb. Two percent of women withdrew from a large-scale clinical trial because of excessive weight gain.
5.12 Carbohydrate Metabolism
A decrease in glucose tolerance has been observed in some patients on Depo-Provera CI treatment. Monitor diabetic patients carefully while receiving Depo-Provera CI.
5.13 Lactation
Detectable amounts of drug have been identified in the milk of mothers receiving Depo-Provera CI. In nursing mothers treated with Depo-Provera CI, milk composition, quality, and amount are not adversely affected. Neonates and infants exposed to medroxyprogesterone from breast milk have been studied for developmental and behavioral effects through puberty. No adverse effects have been noted.
5.14 Fluid Retention
Because progestational drugs including Depo-Provera CI may cause some degree of fluid retention, monitor patients with conditions that might be influenced by this condition, such as epilepsy, migraine, asthma, and cardiac or renal dysfunction.
5.15 Return of Fertility
Return to ovulation and fertility is likely to be delayed after stopping Depo-Provera CI. In a large US study of women who discontinued use of Depo-Provera CI to become pregnant, data are available for 61% of them. Of the 188 women who discontinued the study to become pregnant, 114 became pregnant. Based on Life-Table analysis of these data, it is expected that 68% of women who do become pregnant may conceive within 12 months, 83% may conceive within 15 months, and 93% may conceive within 18 months from the last injection. The median time to conception for those who do conceive is 10 months following the last injection with a range of 4 to 31 months, and is unrelated to the duration of use. No data are available for 39% of the patients who discontinued Depo-Provera CI to become pregnant and who were lost to follow-up or changed their mind.
5.16 Sexually Transmitted Diseases
Patients should be counseled that Depo-Provera CI does not protect against HIV infection (AIDS) and other sexually transmitted diseases.
5.17 Pregnancy
Although Depo-Provera CI should not be used during pregnancy, there appears to be little or no increased risk of birth defects in women who have inadvertently been exposed to medroxyprogesterone acetate injections in early pregnancy. Neonates exposed to medroxyprogesterone acetate in-utero and followed to adolescence showed no evidence of any adverse effects on their health including their physical, intellectual, sexual or social development.
5.18 Monitoring
A woman who is taking hormonal contraceptive should have a yearly visit with her healthcare provider for a blood pressure check and for other indicated healthcare.
5.19 Interference With Laboratory Tests
The use of Depo-Provera CI may change the results of some laboratory tests, such as coagulation factors, lipids, glucose tolerance, and binding proteins. [See Drug Interactions (7.2).]
Find DEPO-PROVERA® CI medical information:
Find DEPO-PROVERA® CI medical information:
DEPO-PROVERA® CI Quick Finder
Health Professional Information
Warnings and Precautions
5 WARNINGS AND PRECAUTIONS
5.1 Loss of Bone Mineral Density
Use of Depo-Provera CI reduces serum estrogen levels and is associated with significant loss of bone mineral density (BMD). This loss of BMD is of particular concern during adolescence and early adulthood, a critical period of bone accretion. It is unknown if use of Depo-Provera CI by younger women will reduce peak bone mass and increase the risk for osteoporotic fracture in later life.
A study to assess the reversibility of loss of BMD in adolescents was conducted with Depo-Provera CI. After discontinuing Depo-Provera CI in these adolescents, mean BMD loss at the total hip and femoral neck did not fully recover by 5 years (60 months) post-treatment in the sub-group of adolescents who were treated for more than 2 years [see Clinical Studies (14.3)]. Similarly, in adults, there was only partial recovery of mean BMD at the total hip, femoral neck, and lumbar spine towards baseline by 2 years post-treatment [see Clinical Studies (14.2)].
The use of Depo-Provera CI is not recommended as a long-term (i.e., longer than 2 years) birth control method unless other options are considered inadequate. BMD should be evaluated when a woman needs to continue to use Depo-Provera CI long-term. In adolescents, interpretation of BMD results should take into account patient age and skeletal maturity.
Other birth control methods should be considered in the risk/benefit analysis for the use of Depo-Provera CI in women with osteoporosis risk factors. Depo-Provera CI can pose an additional risk in patients with risk factors for osteoporosis (e.g., metabolic bone disease, chronic alcohol and/or tobacco use, anorexia nervosa, strong family history of osteoporosis or chronic use of drugs that can reduce bone mass such as anticonvulsants or corticosteroids).
5.2 Thromboembolic Disorders
There have been reports of serious thrombotic events in women using Depo-Provera CI (150 mg). However, Depo-Provera CI has not been causally associated with the induction of thrombotic or thromboembolic disorders. Any patient who develops thrombosis while undergoing therapy with Depo-Provera CI should discontinue treatment unless she has no other acceptable options for birth control.
Do not re-administer Depo-Provera CI pending examination if there is a sudden partial or complete loss of vision or if there is a sudden onset of proptosis, diplopia, or migraine. Do not re-administer if examination reveals papilledema or retinal vascular lesions.
5.3 Cancer Risks
Breast Cancer
Women who have or have had a history of breast cancer should not use hormonal contraceptives, including Depo-Provera CI, because breast cancer may be hormonally sensitive [see Contraindications (4)]. Women with a strong family history of breast cancer should be monitored with particular care.
The results of five large case-control studies assessing the association between depo-medroxyprogesterone acetate (DMPA) use and the risk of breast cancer are summarized in Figure 1. Three of the studies suggest a slightly increased risk of breast cancer in the overall population of users; these increased risks were statistically significant in one study. One recent US study1 evaluated the recency and duration of use and found a statistically significantly increased risk of breast cancer in recent users (defined as last use within the past five years) who used DMPA for 12 months or longer; this is consistent with results of a previous study2.
Figure 1 Risk estimates for breast cancer in DMPA users
| Odds ratio estimates were adjusted for the following covariates: |
| Lee et al. (1987): age, parity, and socioeconomic status. |
| Paul et al. (1989): age, parity, ethnic group, and year of interview. |
| WHO (1991): age, center, and age at first live birth. |
| Shapiro et al. (2000): age, ethnic group, socioeconomic status, and any combined estrogen/progestogen oral contraceptive use. |
| Li et al. (2012): age, year, BMI, duration of OC use, number of full-term pregnancies, family history of breast cancer, and history of screening mammography. |
|
Based on the published SEER-18 2011 incidence rate (age-adjusted to the 2000 US Standard Population) of breast cancer for US women, all races, age 20 to 49 years, a doubling of risk would increase the incidence of breast cancer in women who use Depo-Provera CI from about 72 to about 144 cases per 100,000 women.
Cervical Cancer
A statistically nonsignificant increase in RR estimates of invasive squamous-cell cervical cancer has been associated with the use of Depo-Provera CI in women who were first exposed before the age of 35 years (RR 1.22 to 1.28 and 95% CI 0.93 to 1.70). The overall, nonsignificant relative rate of invasive squamous-cell cervical cancer in women who ever used Depo-Provera CI was estimated to be 1.11 (95% CI 0.96 to 1.29). No trends in risk with duration of use or times since initial or most recent exposure were observed.
5.4 Ectopic Pregnancy
Be alert to the possibility of an ectopic pregnancy among women using Depo-Provera CI who become pregnant or complain of severe abdominal pain.
5.5 Anaphylaxis and Anaphylactoid Reaction
Anaphylaxis and anaphylactoid reaction have been reported with the use of Depo-Provera CI. Institute emergency medical treatment if an anaphylactic reaction occurs.
5.6 Injection Site Reactions
Injection site reactions have been reported with use of Depo-Provera CI [see Adverse Reactions (6.2)]. Persistent injection site reactions may occur after administration of Depo-Provera CI due to inadvertent subcutaneous administration or release of the drug into the subcutaneous space while removing the needle [see Dosage and Administration (2.1)].
5.7 Liver Function
Discontinue Depo-Provera CI use if jaundice or acute or chronic disturbances of liver function develop. Do not resume use until markers of liver function return to normal and Depo-Provera CI causation has been excluded.
5.8 Convulsions
There have been a few reported cases of convulsions in patients who were treated with Depo-Provera CI. Association with drug use or pre-existing conditions is not clear.
5.9 Depression
Monitor patients who have a history of depression and do not re-administer Depo-Provera CI if depression recurs.
5.10 Bleeding Irregularities
Most women using Depo-Provera CI experience disruption of menstrual bleeding patterns. Altered menstrual bleeding patterns include amenorrhea, irregular or unpredictable bleeding or spotting, prolonged spotting or bleeding, and heavy bleeding. Rule out the possibility of organic pathology if abnormal bleeding persists or is severe, and institute appropriate treatment.
As women continue using Depo-Provera CI, fewer experience irregular bleeding and more experience amenorrhea. In clinical studies of Depo-Provera CI, by month 12 amenorrhea was reported by 55% of women, and by month 24, amenorrhea was reported by 68% of women using Depo-Provera CI.
5.11 Weight Gain
Women tend to gain weight while on therapy with Depo-Provera CI. From an initial average body weight of 136 lb, women who completed 1 year of therapy with Depo-Provera CI gained an average of 5.4 lb. Women who completed 2 years of therapy gained an average of 8.1 lb. Women who completed 4 years gained an average of 13.8 lb. Women who completed 6 years gained an average of 16.5 lb. Two percent of women withdrew from a large-scale clinical trial because of excessive weight gain.
5.12 Carbohydrate Metabolism
A decrease in glucose tolerance has been observed in some patients on Depo-Provera CI treatment. Monitor diabetic patients carefully while receiving Depo-Provera CI.
5.13 Lactation
Detectable amounts of drug have been identified in the milk of mothers receiving Depo-Provera CI. In nursing mothers treated with Depo-Provera CI, milk composition, quality, and amount are not adversely affected. Neonates and infants exposed to medroxyprogesterone from breast milk have been studied for developmental and behavioral effects through puberty. No adverse effects have been noted.
5.14 Fluid Retention
Because progestational drugs including Depo-Provera CI may cause some degree of fluid retention, monitor patients with conditions that might be influenced by this condition, such as epilepsy, migraine, asthma, and cardiac or renal dysfunction.
5.15 Return of Fertility
Return to ovulation and fertility is likely to be delayed after stopping Depo-Provera CI. In a large US study of women who discontinued use of Depo-Provera CI to become pregnant, data are available for 61% of them. Of the 188 women who discontinued the study to become pregnant, 114 became pregnant. Based on Life-Table analysis of these data, it is expected that 68% of women who do become pregnant may conceive within 12 months, 83% may conceive within 15 months, and 93% may conceive within 18 months from the last injection. The median time to conception for those who do conceive is 10 months following the last injection with a range of 4 to 31 months, and is unrelated to the duration of use. No data are available for 39% of the patients who discontinued Depo-Provera CI to become pregnant and who were lost to follow-up or changed their mind.
5.16 Sexually Transmitted Diseases
Patients should be counseled that Depo-Provera CI does not protect against HIV infection (AIDS) and other sexually transmitted diseases.
5.17 Pregnancy
Although Depo-Provera CI should not be used during pregnancy, there appears to be little or no increased risk of birth defects in women who have inadvertently been exposed to medroxyprogesterone acetate injections in early pregnancy. Neonates exposed to medroxyprogesterone acetate in-utero and followed to adolescence showed no evidence of any adverse effects on their health including their physical, intellectual, sexual or social development.
5.18 Monitoring
A woman who is taking hormonal contraceptive should have a yearly visit with her healthcare provider for a blood pressure check and for other indicated healthcare.
5.19 Interference With Laboratory Tests
The use of Depo-Provera CI may change the results of some laboratory tests, such as coagulation factors, lipids, glucose tolerance, and binding proteins. [See Drug Interactions (7.2).]
Health Professional Information
{{section_name_patient}}
{{section_body_html_patient}}
Resources
Didn’t find what you were looking for? Contact us.
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine.
*Speak with a Pfizer Medical Information Professional regarding your medical inquiry. Available 9AM-5Pm ET Monday to Friday; excluding holidays.
Submit a medical question for Pfizer prescription products.
Report Adverse Event
To report an adverse event related to the Pfizer-BioNTech COVID-19 Vaccine, and you are not part of a clinical trial* for this product, click the link below to submit your information:
Pfizer Safety Reporting Site*If you are involved in a clinical trial for this product, adverse events should be reported to your coordinating study site.
If you cannot use the above website, or would like to report an adverse event related to a different Pfizer product, please call Pfizer Safety at (800) 438-1985.
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or call (800) 822-7967.