CORLOPAM
(fenoldopam mesylate injection, USP)
Find CORLOPAM medical information:
Find CORLOPAM medical information:
CORLOPAM Quick Finder
Highlights
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use CORLOPAM safely and effectively. See full prescribing information for CORLOPAM. CORLOPAM (fenoldopam mesylate) injection, for intravenous use Initial U.S. Approval: 1997 INDICATIONS AND USAGEFenoldopam injection is a dopaminergic agonist indicated:
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONSNone (4). WARNINGS AND PRECAUTIONS
ADVERSE REACTIONSThe most common events (occurring in more than 5% of patients) reported associated with use are headache, cutaneous dilation (flushing), nausea, and hypotension (6.1). To report SUSPECTED ADVERSE REACTIONS, contact Pfizer Inc. at 1-800-438-1985, or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. DRUG INTERACTIONSBeta-blockers: Avoid concomitant use (7.1). See 17 for PATIENT COUNSELING INFORMATION. Revised: 8/2021 |
Indications and Usage
1 INDICATIONS AND USAGE
1.1 Adult Patients
Fenoldopam is indicated for in-hospital, short-term (up to 48 hours) management of severe hypertension when rapid, but quickly reversible, emergency reduction of blood pressure is clinically indicated, including malignant hypertension with deteriorating end-organ function. Transition to oral therapy with another agent can begin at any time after blood pressure is stable during fenoldopam infusion.
1.2 Pediatric Patients
Fenoldopam is indicated for in-hospital, short-term (up to 4 hours) reduction in blood pressure [see Clinical Pharmacology (12.2)].
Dosage and Administration
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
Adult Patients
Initiate dosing at 0.01 to 0.3 mcg/kg/min as a continuous intravenous infusion. Dosing may be increased in increments of 0.05 to 0.1 mcg/kg/minute every 15 minutes or longer, until target blood pressure is reached [see Clinical Pharmacology (12.2)]; the maximal infusion rate reported in clinical studies was 1.6 mcg/kg/minute. Doses lower than 0.1 mcg/kg/min and slow up-titration have been associated with less reflex tachycardia. Maintenance infusions may be continued for up to 48 hours.
Oral antihypertensive agents can be added during fenoldopam infusion or after discontinuation.
Pediatric Patients
Initiate dosing at 0.2 mcg/kg/minute and titrate dose by 0.3 to 0.5 mcg/kg/min every 20-30 minutes to a maximum dose of 0.8 mcg/kg/minute. Higher doses generally produced no further decreases in Mean Arterial Pressure (MAP) but did worsen tachycardia.
2.2 Preparation and Administration
Dilute contents of vials with 0.9% Sodium Chloride Injection or 5% Dextrose in Water before infusion. Each vial is for single use only. Discard diluted solution if not being administered to a patient after 4 hours at room temperature or 24 hours at refrigerated temperature. Inspect parenteral drug products for particulate matter and discoloration prior to administration, whenever solution and container permit. If particulate matter or cloudiness is observed, discard the drug.
mL of Concentrate (mg of drug) | Added to | Final Concentration |
4 mL (40 mg) | 1000 mL | 40 mcg/mL |
2 mL (20 mg) | 500 mL | 40 mcg/mL |
1 mL (10 mg) | 250 mL | 40 mcg/mL |
mL of Concentrate (mg of drug) | Added to | Final Concentration |
3 mL (30 mg) | 500 mL | 60 mcg/mL |
1.5 mL (15 mg) | 250 mL | 60 mcg/mL |
0.6 mL (6 mg) | 100 mL | 60 mcg/mL |
Rates of infusion in mL/hour for fenoldopam may be calculated using the following formula:
Infusion Rate (mL/h) = [Dose (mcg/kg/min) x Weight (kg) x 60 min/h]
Concentration (mcg/mL)
Example calculations for infusion rates are as follows:
Example 1: for a 60 kg patient at an initial dose of 0.01 mcg/kg/min using a 40 mcg/mL concentration, the infusion rate would be as follows:
Infusion Rate (mL/h) = [0.01 (mcg/kg/min) x 60 (kg) x 60 (min/h)] = 0.9 (mL/h)
40 (mcg/mL)
Example 2: for a 10 kg patient at a dose of 0.2 mcg/kg/min using a 60 mcg/mL concentration, the infusion rate would be as follows:
Infusion Rate (mL/h) = [0.2 (mcg/kg/min) x 10 (kg) x 60 (min/h)] = 2.0 (mL/h)
60 (mcg/mL)
Dosage Forms and Strengths
Contraindications
Warnings and Precautions
5 WARNINGS AND PRECAUTIONS
5.1 Tachycardia
Fenoldopam causes a dose-related tachycardia, particularly with infusion rates above 0.1 mcg/kg/min in adults and >0.8 mcg/kg/min in pediatric patients. Tachycardia in adults may diminish with continued therapy at doses of fenoldopam of <0.1 mcg/kg/min.
5.2 Hypokalemia
Hypokalemia has been observed after less than 6 hours of fenoldopam infusion. Hypokalemia reflects a pressure natriuresis with enhanced potassium-sodium exchange or a direct drug effect. Monitor serum potassium levels.
5.3 Increased Intraocular Pressure
In a clinical study of 12 patients with open-angle glaucoma or ocular hypertension (mean baseline intraocular pressure was 29.2 mm Hg with a range of 22 to 33 mm Hg), infusion of fenoldopam at escalating doses ranging from 0.05 to 0.5 mcg/kg/min over a 3.5 hour period caused a dose-dependent increase in intraocular pressure (IOP). At the peak effect, the intraocular pressure was raised by a mean of 6.5 mm Hg (range -2 to +8.5 mm Hg, corrected for placebo effect). Upon discontinuation of the fenoldopam infusion, the IOP returned to baseline values within 2 hours.
5.4 Allergic Reactions Associated with Sulfite
Contains sodium metabisulfite, a sulfite that may cause allergic-type reactions including anaphylactic symptoms and life-threatening or less severe asthmatic episodes in certain susceptible people. The overall prevalence of sulfite sensitivity in the general population is unknown and probably low. Sulfite sensitivity is seen more frequently in asthmatic than in non-asthmatic people.
Adverse Reactions
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The most common reactions associated with fenoldopam use are headache, cutaneous dilation (flushing), nausea, and hypotension, each reported in more than 5% of patients.
Adverse reactions occurring more than once in any dosing group (once if potentially important or plausibly drug-related) in the fixed-dose constant-infusion studies are presented in Table 3. There was no clear dose relationship, except possibly for headache, nausea, flushing.
Event | Placebo (n = 7) | Fenoldopam (n = 125) |
n (%) | n (%) | |
Headache | 1 (14%) | 30 (24%) |
Nausea | 0 | 15 (12%) |
Vomiting | 0 | 7 (6%) |
Injection site reaction | 0 | 9 (7%) |
Electrocardiogram T wave inversion | 0 | 7 (6%) |
The following additional adverse reactions were observed more frequently in patients treated with fenoldopam
Incidence 0.5% to 5%
Metabolism and Nutrition Disorders — Hypokalemia
Psychiatric Disorders — Nervousness/Anxiety, insomnia
Nervous System Disorders — Dizziness
Cardiac Disorders — Extrasystoles, palpitations, cardiac failure, ischemic heart disease, myocardial infarction, angina pectoris, tachycardia
Gastrointestinal Disorders — Abdominal pain
Skin and Subcutaneous Tissue Disorders — Hyperhidrosis
Musculoskeletal and Connective Tissue Disorders —Muscle spasms
Renal and Urinary Disorders — Oliguria
General Disorders and Administration Site Conditions —Chest pain, pyrexia
Investigations — Blood urea increased, blood creatinine increased, blood glucose increased, transaminases increased, blood lactate dehydrogenase increased
6.2 Post-Marketing Experience
The following adverse reactions have been identified during post approval use of CORLOPAM. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. Voluntary reports of adverse reactions temporally associated with CORLOPAM that have been received since market introduction include the following:
Cardiac Disorders — Cardiogenic shock
Vascular Disorders — Hypotension
Gastrointestinal Disorders — Abdominal distension
Investigations — Electrocardiogram ST segment depression, oxygen saturation decreased
Drug Interactions
Use in Specific Populations
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are insufficient data regarding CORLOPAM use in pregnancy to evaluate for a drug-associated risk of major birth defects, miscarriage or adverse maternal or fetal outcomes.
In animal studies, there was no evidence of teratogenicity or fetotoxicity when fenoldopam was orally administered to rats and rabbits during organogenesis (see Data). There are adverse effects on maternal and fetal outcomes associated with severe hypertension (see Clinical Considerations).
The estimated background risk for major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in the clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Data
Animal Data
Oral reproduction studies have been performed in rats and rabbits at doses of 12.5 to 200 mg/kg/day and 6.25 to 25 mg/kg/day, respectively, administered during the period of organogenesis. Studies have revealed maternal toxicity at the highest doses tested but no evidence of harm to the fetus due to fenoldopam.
8.2 Lactation
Risk Summary
There are no data on the presence of fenoldopam in human milk, the effects on the breastfed child, or the effects on milk production. Fenoldopam is present in rat milk. When a drug is present in animal milk, it is likely that the drug will be present in human milk.
Because of the potential for severe adverse reactions in the breastfed infant, advise women not to breastfeed during treatment with CORLOPAM.
8.4 Pediatric Use
Safety and effectiveness of fenoldopam have been established in the age groups age < 1 month (at least 2 kg or full term) to 12 years old requiring blood pressure reduction [see Clinical Pharmacology (12.2)]. The adverse event profile in pediatric patients is similar to that seen in adults.
The pharmacokinetics of fenoldopam are independent of age when corrected for body weight.
The long-term effects of fenoldopam on growth and development have not been studied.
8.5 Geriatric Use
Clinical studies of fenoldopam did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should start at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Overdosage
Description
11 DESCRIPTION
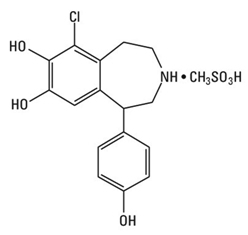
CORLOPAM (Fenoldopam Mesylate Injection, USP) is a dopamine D1-like receptor agonist. The product is formulated as a solution to be diluted for intravenous infusion. Chemically it is 6-chloro-2,3,4,5-tetrahydro-1-(4-hydroxyphenyl)-[1H]-3-benzazepine-7,8-diol methanesulfonate with the following structure:
fenoldopam mesylate
Fenoldopam mesylate is a white to off-white powder with a molecular weight of 401.87 and a molecular formula of C16H16ClNO3•CH3SO3H. It is sparingly soluble in water, ethanol and methanol, and is soluble in propylene glycol.
Each 1 mL contains, in sterile aqueous solution, citric acid 3.44 mg; fenoldopam mesylate equivalent to fenoldopam 10 mg; propylene glycol 518 mg; sodium citrate dihydrate 0.61 mg; sodium metabisulfite 1 mg.
Clinical Pharmacology
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Fenoldopam is a rapid-acting vasodilator. It is an agonist for D1-like dopamine receptors and binds with moderate affinity to α2-adrenoceptors. It has no significant affinity for D2-like receptors, α1 and β-adrenoceptors, 5HT1 and 5HT2 receptors, or muscarinic receptors. Fenoldopam is a racemic mixture with the R-isomer responsible for the biological activity. The R-isomer has approximately 250-fold higher affinity for D1-like receptors than does the S-isomer. In non-clinical studies, fenoldopam had no agonist effect on presynaptic D2-like dopamine receptors, or α- or β-adrenoceptors, nor did it affect angiotensin-converting enzyme activity. Fenoldopam may increase norepinephrine plasma concentration.
In animals, fenoldopam has vasodilating effects in coronary, renal, mesenteric and peripheral arteries. All vascular beds, however, do not respond uniformly to fenoldopam. Vasodilating effects have been demonstrated in renal efferent and afferent arterioles.
12.2 Pharmacodynamics
Mild to Moderate Hypertension
In a randomized double-blind, placebo-controlled, 5-group study in 32 patients with mild to moderate essential hypertension (diastolic blood pressure between 95 and 119 mm Hg), and a mean baseline pressure of about 154/98 mm Hg, and heart rate of about 75 bpm, fixed-rate IV infusions of fenoldopam produced dose-related reductions in systolic and diastolic blood pressures. Infusions were maintained at a fixed rate for 48 hours. Table 4 shows the results of the study. The onset of response was rapid at all infusion rates, with the 15-minute response representing 50 to 100% of the 1 hour response in all groups. There was some suggestion of partial tolerance at 48 hours in the 2 higher dose infusions, but a substantial effect persisted through 48 hours. When infusions were stopped, blood pressure gradually returned to pretreatment values with no evidence of rebound. This study suggests that there is no greater response to 0.8 mcg/kg/min than to 0.4 mcg/kg/min.
Drug Dosage (mcg/kg/min) | |||||
Placebo n = 7 | 0.04 n = 7 | 0.1 n = 7 | 0.4 n = 5 | 0.8 n = 6 | |
15 Minutes of Infusion* | |||||
Systolic BP (mmHg) | 0 ± 6 | -15 ± 6 | -19 ± 8 | -14 ± 4 | -24 ± 6 |
Diastolic BP (mmHg) | 0 ± 2 | -5 ± 3 | -12 ± 4 | -15 ± 3 | -20 ± 4 |
Heart rate (bpm) | +2 ± 2 | +3 ± 2 | +5 ± 1 | +16 ± 3 | +19 ± 3 |
30 Minutes of Infusion* | |||||
Systolic BP | -6 ± 5 | -17 ± 6 | -18 ± 6 | -14 ± 8 | -26 ± 6 |
Diastolic BP | -6 ± 3 | -7 ± 3 | -16 ± 4 | -14 ± 3 | -20 ± 2 |
Heart rate | +2 ± 2 | +3 ± 2 | +10 ± 2 | +18 ± 3 | +23 ± 3 |
1 Hour of Infusion* | |||||
Systolic BP | -15 ± 4 | -22 ± 7 | -22 ± 7 | -26 ± 9 | -22 ± 9 |
Diastolic BP | -5 ± 3 | -9 ± 2 | -18 ± 4 | -19 ± 4 | -21 ± 1 |
Heart rate | +1 ± 3 | +5 ± 2 | +12 ± 3 | +19 ± 4 | +25 ± 4 |
4 Hours of Infusion* | |||||
Systolic BP | -14 ± 5 | -16 ± 9 | -31 ± 15 | -22 ± 11 | -25 ± 7 |
Diastolic BP | -14 ± 8 | -8 ± 4 | -19 ± 9 | -25 ± 3 | -20 ± 1 |
Heart rate | +5 ± 3 | +6 ± 3 | +10 ± 4 | +21 ± 2 | +27 ± 7 |
24 Hours of Infusion* | |||||
Systolic BP | -20 ± 6 | -23 ± 8 | -35 ± 7 | -22 ± 6 | -23 ± 11 |
Diastolic BP | -11 ± 6 | -11 ± 5 | -23 ± 10 | -22 ± 5 | -13 ± 3 |
Heart rate | +6 ± 3 | +5 ± 3 | +13 ± 2 | +17 ± 4 | +15 ± 3 |
48 Hours of Infusion* | |||||
Systolic BP | -12 ± 8 | -31 ± 6 | -22 ± 8 | -9 ± 6 | -14 ±10 |
Diastolic BP | -9 ± 5 | -10 ± 6 | -9 ± 7 | -9 ± 2 | -9 ± 3 |
Heart rate | +1 ± 2 | 0 ± 4 | +1 ± 4 | +12 ± 3 | +8 ± 3 |
Hypertensive Emergencies
In a multicenter, randomized, double-blind comparison of four infusion rates, fenoldopam was administered as constant rate infusions of 0.01, 0.03, 0.1 and 0.3 mcg/kg/min for up to 24 hours to 94 adult patients experiencing hypertensive emergencies (defined as diastolic blood pressure ≥120 mmHg with evidence of compromise of end-organ function involving the cardiovascular, renal, cerebral or retinal systems). Infusion rates could be doubled after one hour if clinically indicated. There were dose-related, rapid-onset, decreases in systolic and diastolic blood pressures and increases in heart rate (Table 5).
Drug Dosage mcg/kg/min | ||||
0.01 n = 25 | 0.03 n = 24 | 0.1 n = 22 | 0.3 n = 23 | |
Pre-Infusion Baseline | ||||
Systolic BP (mmHg) | 210 ± 21 | 208 ± 26 | 205 ± 24 | 211 ± 17 |
Diastolic BP (mmHg) | 136 ± 16 | 135 ± 11 | 133 ± 14 | 136 ± 15 |
Heart rate (bpm) | 87 ± 20 | 84 ± 14 | 81 ± 19 | 80 ± 14 |
15 minutes of Infusion | ||||
Systolic BP | -5 ± 4 | -7 ± 4 | -16 ± 4 | -19 ± 4 |
Diastolic BP | -5 ± 3 | -8 ± 3 | -12 ± 2 | -21 ± 2 |
Heart rate | -2 ± 3 | +1 ± 1 | +2 ± 1 | +11 ± 2 |
30 Minutes of Infusion | ||||
Systolic BP | -6 ± 4 | -11 ± 4 | -21 ± 3 | -16 ± 4 |
Diastolic BP | -10 ± 3 | -12 ± 3 | -17 ± 3 | -20 ± 2 |
Heart rate | -2 ± 3 | -1 ± 1 | +3 ± 2 | +12 ± 3 |
1 Hour of Infusion | ||||
Systolic BP | -5 ± 3 | -9 ± 4 | -19 ± 4 | -22 ± 4 |
Diastolic BP | -8 ± 3 | -13 ± 3 | -18 ± 2 | -23 ± 2 |
Heart rate | -1 ± 3 | 0 ± 2 | +3 ± 2 | +11 ± 3 |
4 Hours of Infusion | ||||
Systolic BP | -14 ± 4 | -20 ± 5 | -23 ± 4 | -37 ± 4 |
Diastolic BP | -12 ± 3 | -18 ± 3 | -21 ± 3 | -29 ± 3 |
Heart rate | -2 ± 4 | 0 ± 2 | +4 ± 2 | +11 ± 2 |
Severe Hypertension
Two hundred thirty-six (236) severely hypertensive adult patients (DBP ≥120 mmHg), with or without end-organ compromise, were randomized to receive in 2 open-label studies either fenoldopam or nitroprusside. The response rate was 79% (92/117) in the fenoldopam group and 77% (90/119) in the nitroprusside group. Response required a decline in supine diastolic blood pressure to less than 110 mmHg if the baseline were between 120 and 150 mmHg, inclusive, or by ≥40 mmHg if the baseline were ≥150 mmHg. Patients were titrated to the desired effect. For fenoldopam, the dose ranged from 0.1 to 1.5 mcg/kg/min; for nitroprusside, the dose ranged from 1 to 8 mcg/kg/min. As in the study in mild to moderate hypertensives, most of the effect seen at 1 hour is present at 15 minutes. The additional effect seen after 1 hour occurs in all groups and may not be drug-related (there was no placebo group for evaluation).
Hypertension in Pediatric Patients
In a randomized, multi-center, double-blind, placebo-controlled, dose-ranging study, pediatric patients were randomized in equal proportions to 1 of 5 treatment groups:
0.05, 0.2, 0.8, or 3.2 mcg/kg/min fenoldopam or placebo. Fenoldopam or placebo was administered as a blinded continuous IV infusion for 30 minutes. Following this, open-label titration of fenoldopam was given to induce hypotension or normotension (defined as MAP, between 50 and 80 mmHg for patients >1 month of age and MAP between 40 and 70 mmHg for patients ≤1 month). Seventy-seven pediatric patients (up to 12 years of age – Tanner Stages 1 and 2) were treated for at least two hours. Of these, 2 were <1 month of age, 25 were between 1 month of age and 1 year of age, 7 were between 1 and 2 years of age, and 43 were between 2 and 12 years of age. Of the 77 patients enrolled in the trial, 58 were enrolled in association with surgery, and 19 were treated in an ICU setting.
The lowest dosage at which decreases in MAP were seen during blinded administration was 0.2 mcg/kg/min. The dose at which the maximum effect was seen was 0.8 mcg/kg/min. Doses higher than 0.8 mcg/kg/min generally produced no further decreases in MAP but did worsen tachycardia (Table 6). Changes in blood pressure and heart rate occurred as early as 5 minutes after starting infusion. Doses as high as 4 mcg/kg/min were administered during the open-label period. The effects increased with time for 15 to 25 minutes, and an effect could still be detected after an average of 4 hours of infusion. When the infusion was discontinued, blood pressure and heart rates approached baseline values during the following 30 minutes.
| |||||
Drug Dosage (mcg/kg/min) | |||||
Placebo | 0.05 | 0.2 | 0.8 | 3.2 | |
n = 16 | n = 15* | n = 16 | n = 15 | n = 15 | |
Pre-Infusion Baseline | |||||
Mean Arterial Pressure | 81 ± 4 | 77 ± 5 | 76 ± 4 | 88 ± 6 | 74 ± 4 |
Systolic BP | 108 ± 5 | 103 ± 6 | 104 ± 6 | 117 ± 7 | 98 ± 4 |
Diastolic BP | 62 ± 4 | 61 ± 4 | 57 ± 3 | 69 ± 6 | 56 ± 3 |
Heart rate | 106 ± 8 | 110 ± 7 | 119 ± 7 | 125 ± 6 | 122 ± 6 |
Change at 5 Minutes of Infusion | |||||
Mean Arterial Pressure | 4 ± 2 | 3 ± 3 | -2 ± 2 | -3 ± 3 | -6 ± 3 |
Systolic BP | 5 ± 3 | 3 ± 3 | -2 ± 3 | -5 ± 3 | -8 ± 3 |
Diastolic BP | 4 ± 2 | 6 ± 2 | -1 ± 2 | -2 ± 2 | -4 ± 2 |
Heart rate | 2 ± 3 | -2 ± 3 | -1 ± 3 | 4 ± 3 | -2 ± 3 |
Change at 30 Minutes of Infusion | |||||
Mean Arterial Pressure | 0 ± 3 | -1 ± 3 | -2 ± 3 | -10 ± 3 | -10 ± 3 |
Systolic BP | -3 ± 4 | 0 ± 4 | -3 ± 4 | -12 ± 4 | -10 ± 4 |
Diastolic BP | 0 ± 3 | 1 ± 3 | -2 ± 3 | -8 ± 3 | -6 ± 3 |
Heart rate | -6 ± 4 | -4 ± 4 | 5 ± 4 | 7 ± 4 | 14 ± 4 |
12.3 Pharmacokinetics
Adult Patients
Fenoldopam, administered as a constant infusion at dosages of 0.01 to 1.6 mcg/kg/min, produced steady-state plasma concentrations that were proportional to infusion rates. The elimination half-life was about 5 minutes in mild to moderate hypertensives, with little difference between the R (active) and S isomers. Steady state concentrations are attained in about 20 minutes (4 half-lives). The steady state plasma concentrations of fenoldopam, at comparable infusion rates, were similar in normotensive patients and in patients with mild to moderate hypertension or hypertensive emergencies.
The pharmacokinetics of fenoldopam were not influenced by age, gender, or race in adult patients with a hypertensive emergency. There have been no formal drug-drug interaction studies using intravenous fenoldopam. Clearance of parent (active) fenoldopam is not altered in adult patients with end-stage renal disease on continuous ambulatory peritoneal dialysis (CAPD) and is not altered in adult patients with severe hepatic failure. The effects of hemodialysis on the pharmacokinetics of fenoldopam have not been evaluated.
Pediatric Patients
In children, aged 1 month to 12 years old, steady-state fenoldopam plasma concentrations were proportional to dose (0.05 mcg/kg/min to 3.2 mcg/kg/min). The elimination half-life and clearance were 3 to 5 minutes and 3 L/h/kg, respectively.
In radiolabeled studies in rats, no more than 0.005% of fenoldopam crossed the blood-brain barrier.
Excretion and Metabolism
Radiolabeled studies show that about 90% of infused fenoldopam is eliminated in urine, 10% in feces. Elimination is largely by conjugation, without participation of cytochrome P-450 enzymes. The principal routes of conjugation are methylation, glucuronidation, and sulfation. Only 4% of the administered dose is excreted unchanged. Animal data indicate that the metabolites are inactive.
Nonclinical Toxicology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
In a 24-month study, mice treated orally with fenoldopam at 12.5, 25, or 50 mg/kg/day, reduced to 25 mg/kg/day on day 209 of study, showed no increase above controls in the incidence of neoplasms. Female mice in the highest dose group had an increased incidence and degree of severity of a fibro-osseous lesion of the sternum compared with control or low-dose animals. Compared to controls, female mice in the middle- and upper-dose groups had a higher incidence and degree of severity of chronic nephritis. These pathologic lesions were not seen in male mice treated with fenoldopam.
In a 24-month study, rats treated orally with fenoldopam at 5, 10 or 20 mg/kg/day, with the mid- and high-dose groups increased to 15 or 25 mg/kg/day, respectively, on day 372 of the study, showed no increase above controls in the incidence or type of neoplasms. Compared with the controls, rats in the mid- and high-dose groups had a higher incidence of hyperplasia of collecting duct epithelium at the tip of the renal papilla.
Fenoldopam did not induce bacterial gene mutation in the Ames test or mammalian gene mutation in the Chinese hamster ovary (CHO) cell assay. In the in vitro chromosomal aberration assay with CHO cells, fenoldopam was associated with statistically significant and dose-dependent increases in chromosomal aberrations, and in the proportion of aberrant metaphases. However, no chromosomal damage was seen in the in vivo mice micronucleus or bone marrow assays.
Oral fertility and general reproduction performance studies in male and female rats at 12.5, 37.5 or 75 mg/kg/day revealed no impairment of fertility or reproduction performance due to fenoldopam.
13.2 Animal Toxicology and/or Pharmacology
Unusual toxicologic findings (arterial lesions in the rat) with fenoldopam are summarized below. These findings have not been observed in mice or dogs. No evidence of a similar lesion in humans has been observed.
Arterial lesions characterized by medial necrosis and hemorrhage have been seen in renal and splanchnic arteries of rats given fenoldopam mesylate by continuous intravenous infusion at doses of 1 to 100 mcg/kg/min for 24 hours. The incidence of these lesions is dose related. Arterial lesions morphologically identical to those observed with fenoldopam have been reported in rats infused with dopamine. Data suggest that the mechanism for this injury involves activation of D1-like dopaminergic receptors. Such lesions have not been seen in dogs given doses up to 100 mcg/kg/min by continuous intravenous infusion for 24 hours, nor were they seen in dogs infused at the same dose for 6 hours daily for 24 days. The clinical significance of this finding is not known.
Oral administration of fenoldopam doses of 10 to 15 mg/kg/day or 20 to 25 mg/kg/day to rats for 24 months induced a higher incidence of polyarteritis nodosa compared to controls. Such lesions were not seen in rats given 5 mg/kg/day of fenoldopam or in mice given the drug at doses up to 50 mg/kg/day for 24 months.
How Supplied/Storage and Handling
Medication Guide
17 PATIENT COUNSELING INFORMATION
- •
- Advise patients with underlying hypertension that they require continued follow up for their medical condition, and, if applicable, encourage patients to continue taking their oral antihypertensive medication(s) as directed.
- •
- Advise patients to contact a healthcare professional immediately for any of the following signs of a new hypertensive emergency: neurological symptoms, visual changes, or evidence of congestive heart failure.
Other
17 PATIENT COUNSELING INFORMATION
17 PATIENT COUNSELING INFORMATION
- •
- Advise patients with underlying hypertension that they require continued follow up for their medical condition, and, if applicable, encourage patients to continue taking their oral antihypertensive medication(s) as directed.
- •
- Advise patients to contact a healthcare professional immediately for any of the following signs of a new hypertensive emergency: neurological symptoms, visual changes, or evidence of congestive heart failure.
Full Patient Information
Full Patient Information
17 PATIENT COUNSELING INFORMATION
- •
- Advise patients with underlying hypertension that they require continued follow up for their medical condition, and, if applicable, encourage patients to continue taking their oral antihypertensive medication(s) as directed.
- •
- Advise patients to contact a healthcare professional immediately for any of the following signs of a new hypertensive emergency: neurological symptoms, visual changes, or evidence of congestive heart failure.
Resources
Didn’t find what you were looking for?
Contact us.
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine.
*Speak with a Pfizer Medical Information Professional regarding your medical inquiry. Available 9AM-5Pm ET Monday to Friday; excluding holidays.
Submit a medical question for Pfizer prescription products.
Report Adverse Event
To report an adverse event related to a Pfizer product and you are not part of a clinical trial* for this product, click the link below to submit your information: Pfizer Safety Reporting Site
*If you are involved in a clinical trial for either product, adverse events should be reported to your coordinating study site.
If you cannot use the above website to report an adverse event related to a Pfizer product, please call Pfizer Medical Information at (800) 438-1985.
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or by calling (800)-332-1088.