CAVERJECT® IMPULSE
(alprostadil)
Find CAVERJECT® IMPULSE medical information:
Find CAVERJECT® IMPULSE medical information:
CAVERJECT® IMPULSE Quick Finder
Highlights
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use CAVERJECT IMPULSE safely and effectively. See full prescribing information for CAVERJECT IMPULSE. CAVERJECT IMPULSE (alprostadil) for injection, for intracavernosal use Initial U.S. Approval: 1981 INDICATIONS AND USAGEDOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHSFor injection: 10 mcg or 20 mcg freeze-dried powder for reconstitution in a dual-chamber syringe (3). CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONSMost common (≥10%) adverse reactions: penile pain (6.1). To report SUSPECTED ADVERSE REACTIONS, contact Pfizer at (1-800-438-1985 and www.pfizer.com) or FDA at 1-800-FDA-1088 or http://www.fda.gov/medwatch. See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling. Revised: 12/2022 |
Indications and Usage
Dosage and Administration
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosage and Administration Instructions
- •
- Alprostadil is available in different strengths and presentations. Determine the most suitable dose and presentation for each patient. Use a new syringe for each dose of CAVERJECT.
- •
- Titrate the dose of CAVERJECT IMPULSE for each patient to the lowest effective dose.
- •
- CAVERJECT IMPULSE doses greater than 60 mcg are not recommended.
- •
- Administer the first doses of CAVERJECT IMPULSE in the health care provider’s office by medically trained personnel.
- •
- Instruct the patient on proper use and assess that they are well trained in the self-injection technique prior to initiation of home use. Refer to the Patient Information and Instructions for Use.
- •
- Re-evaluate patients regularly (every 3 months or as clinically appropriate) and determine if dosage adjustments are needed.
2.2 Recommended Dosage for Erectile Dysfunction
Erectile Dysfunction of Vasculogenic, Psychogenic, or Mixed Etiology.
- •
- Initiate dosing with 2.5 mcg of CAVERJECT IMPULSE intracavernousally as recommended [see Dosage and Administration 2.4].
- •
- If there is a partial response at 2.5 mcg, administer another dose of 2.5 mcg within 1 hour.
- •
- During titration, no more than 2 doses should be given within a 24-hour period.
- •
- If additional titration is required, administer doses in increments of 5 to 10 mcg at least 24 hours apart.
- •
- The optimal dose should produce an erection suitable for intercourse that does not exceed a duration of 1 hour.
- •
- The patient must stay in the health care provider’s office until complete detumescence occurs.
Repeat the titration as necessary until the optimal dose is achieved. Doses greater than 60 mcg are not recommended.
Erectile Dysfunction of Pure Neurogenic Etiology (Spinal Cord Injury):
- •
- Initiate dosing with 1.25 mcg of alprostadil using CAVERJECT.
- •
- If there is a partial response, administer another dose of CAVERJECT of 1.25 mcg within 1 hour.
- •
- No more than 2 doses during initial titration should be given within a 24-hour period.
- •
- If additional titration is required, administer a dose of 5 mcg at least 24 hours later.
- •
- The optimal dose should produce an erection suitable for intercourse that does not exceed a duration of 1 hour.
- •
- The patient must stay in the health care provider’s office until complete detumescence occurs.
Repeat the titration as necessary until the optimal dose is achieved. Doses greater than 60 mcg are not recommended.
Maintenance Dosage for Patient Home Use:
- •
- Once the dose of CAVERJECT IMPULSE has been determined in the health care provider’s office, additional dose adjustment may be required after consultation with the health care provider. Adjust the dose in accordance with the titration guidelines described above.
- •
- The recommended frequency of injection is no more than 3 times weekly, with at least 24 hours between each dose.
Adjunct to the Diagnosis of Erectile Dysfunction
To diagnose erectile dysfunction (pharmacologic testing), inject CAVERJECT IMPULSE intracavernosally and monitor patients for the occurrence of an erection. Extensions of this testing are the use of CAVERJECT as an adjunct to laboratory investigations, such as duplex or Doppler imaging. For any of these tests, use a single dose of CAVERJECT IMPULSE that induces a rigid erection. Use the dose regimen for ‘Erectile Dysfunction of Vasculogenic, Psychogenic, or Mixed Etiology’ above.
2.3 Syringe Preparation Instructions
- 1.
- Select the CAVERJECT IMPULSE syringe based upon dose to be administered.
| Syringe Strength | Reconstituted Concentration | Dosages Available for Delivery after Reconstitution | |||
|---|---|---|---|---|---|
10 mcg | 10 mcg/0.5 mL | 2.5 mcg | 5 mcg | 7.5 mcg | 10 mcg |
20 mcg | 20 mcg/0.5 mL | 5 mcg | 10 mcg | 15 mcg | 20 mcg |
- 2.
- Open the sealed plastic tray. Remove the syringe, the needle assembly, and the alcohol swabs from the tray. The syringe has a dose window and a plunger. The needle assembly is a sealed unit that contains the outer protective cap, the inner protective cap, and the superfine needle.
- 3.
- Use the alcohol swab to wipe the rubber membrane at the tip of the syringe. Pick up the needle assembly, grasp the paper tab, and peel off the paper cover (the lid).
- 4.
- Hold the needle assembly by the cap and press the needle assembly onto the tip of the syringe. Turn it clockwise until the needle assembly is firmly locked into place.
- 5.
- Remove the outer protective cap from the needle by twisting it clockwise. Do not yet remove the inner protective cap, the thin plastic tube that directly covers the needle.
- 6.
- Hold the syringe system with the needle pointing upward. The plunger rod should still be in the fully extended position, with all of the threads visible. Slowly rotate the plunger rod clockwise until it goes all the way in and stops. Do not push on the plunger while trying to rotate it.
- 7.
- Turn the syringe upside down several times to make sure the solution is evenly mixed. The solution should be clear. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration. The product should not be used if particulate matter or discoloration are present.
- 8.
- Hold the syringe with the needle upward and carefully remove the inner protective cap from the needle. Lightly tap the glass cartridge a few times with your finger until any large bubbles disappear up into the tip. With the syringe pointed upward, push in the plunger rod until it stops to push any air out.
- 9.
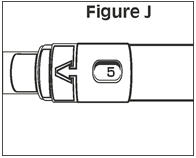
- To set the dose: locate the dose window on the syringe and then slowly turn the plunger rod clockwise until the correct dose number appears in the center of the window. The syringe is now ready for use. If you pass the correct number, keep turning the plunger in the same direction until the correct number comes around again – do not try to turn it backward.
- 10.
- After reconstitution, the syringe should be used within 24 hours when stored between 36 to 77°F (2°C to 25°C). Do not freeze. CAVERJECT IMPULSE is for single use only. Discard the injection delivery system and any remaining solution after use.
2.4 Administration Instructions
- •
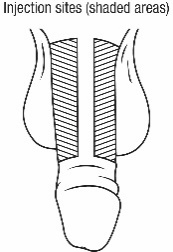
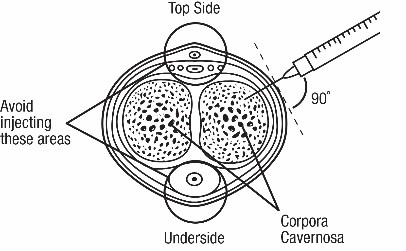
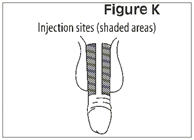
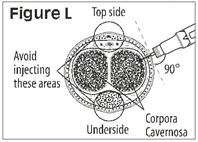
- Administer CAVERJECT IMPUSLE intracavernosally along the dorso-lateral aspect of the proximal third of the penis. See Figures A and B below.
Figure A
Figure B
- •
- Wipe the intended injection site with an alcohol swab prior to injection.
- •
- Avoid visible veins during injection.
- •
- Alternate the side of the penis that is injected and the site of injection.
- •
- Compress the site of injection with an alcohol swab or sterile gauze for 5 minutes.
- •
- Each CAVERJECT IMPULSE syringe is intended for single use only (one dose only) and should be discarded after use.
Dosage Forms and Strengths
3 DOSAGE FORMS AND STRENGTHS
Caverject Impulse (alprostadil) for injection contains sterile, freeze-dried alprostadil for reconstitution and sterile bacteriostatic water in a prefilled dual chamber glass cartridge. It is available in 10 mcg and 20 mcg strengths.
For injection: 10 mcg and 20 mcg of alprostadil freeze-dried powder for reconstitution in single-dose, pre‑filled, dual chambered glass cartridge syringe. The front chamber contains alprostadil and the rear chamber contains sterile bacteriostatic water. A 29 gauge needle system is also included.
The reconstituted solution is clear.
Contraindications
4 CONTRAINDICATIONS
CAVERJECT IMPULSE is contraindicated:
- •
- in men who have a known hypersensitivity to the drug [see Adverse Reactions (6.1)]
- •
- in men who have conditions that predispose them to priapism, such as sickle cell anemia or sickle cell trait, multiple myeloma, or leukemia [see Warnings and Precautions (5.1)]
- •
- for the treatment of erectile dysfunction in men with fibrotic conditions of the penis, such as anatomical deformation, angulation, cavernosal fibrosis, or Peyronie's disease [see Warnings and Precautions (5.2)]
- •
- in men with penile implants.
Warnings and Precautions
5 WARNINGS AND PRECAUTIONS
5.1 Prolonged Erection and Priapism
Prolonged erection, defined as erection lasting between 4 to 6 hours in duration, occurred in 4% of 1,861 patients treated up to 18 months in studies of CAVERJECT. The incidence of priapism (erections lasting more than 6 hours in duration) was 0.4%. In the event of an erection that persists longer than 4 hours, the patient should seek immediate medical assistance. If priapism is not treated immediately, penile tissue damage and permanent loss of potency may result.
To minimize the chances of prolonged erection or priapism, titrate CAVERJECT IMPULSE to the lowest effective dose [see Dosage and Administration (2.1]. In addition, do not use CAVERJECT IMPULSE in patients who have conditions that predispose them to priapism, such as sickle cell anemia or sickle cell trait, multiple myeloma, or leukemia [see Contraindications (4)].
5.2 Penile Fibrosis
The overall incidence of penile fibrosis reported in clinical studies with CAVERJECT was 3%. In one self-injection clinical study where duration of use was up to 18 months, the incidence of penile fibrosis was 7.8%.
Physical examination of the penis should be performed periodically to detect signs of penile fibrosis. Treatment with CAVERJECT IMPULSE should be discontinued in patients who develop penile angulation or cavernosal fibrosis.
5.3 Hypotension
Intracavernous injections of CAVERJECT IMPULSE can increase peripheral blood levels of alprostadil which can result in hypotension. Avoid use of CAVERJECT IMPULSE in patients with known cavernosal venous leakage.
5.4 Injection Site Bleeding When Used with Anticoagulants
Patients on anticoagulants, such as warfarin or heparin, may have increased propensity for injection site bleeding after intracavernosal injection with CAVERJECT IMPULSE. Compress the site of injection with an alcohol swab or sterile gauze for 5 minutes.
5.5 Cardiovascular Risk Related to Underlying Medical Conditions
There is a potential for cardiac risk of sexual activity in patients with preexisting cardiovascular disease. Therefore, treatments for erectile dysfunction, including CAVERJECT IMPULSE, generally should not be used in men for whom sexual activity is inadvisable because of their underlying cardiovascular status. In addition, the evaluation of erectile dysfunction should include a determination of potential underlying causes and the identification of appropriate treatment following a complete medical assessment.
5.6 Risks of Use in Combination with Other Vasoactive Medications Injected Intracavernosally
The safety and efficacy of combinations of CAVERJECT IMPULSE and other vasoactive agents injected intracavernosally have not been established in clinical studies. The risks of prolonged erection, priapism, and hypotension may be increased.
5.7 Needle Breakage
CAVERJECT IMPULSE uses a superfine (29 gauge) needle for administration. As with all superfine needles, the possibility of needle breakage exists. Needle breakage, with a portion of the needle remaining in the penis, has been reported and, in some cases, has required hospitalization and surgical removal. Careful instruction in proper patient handling and injection techniques may minimize the potential for needle breakage [see Dosage and Administration (2.3) and Adverse Reactions (6.2)].
5.8 Risk of Serious Adverse Reactions in Infants due to Benzyl Alcohol
The preservative benzyl alcohol contained in CAVERJECT IMPULSE has been associated with serious adverse events, including the "gasping syndrome", and death in pediatric patients. The minimum amount of benzyl alcohol at which toxicity may occur is not known. The risk of benzyl alcohol toxicity depends on the quantity administered and the liver and kidneys' capacity to detoxify the chemical. Premature and low-birth weight infants may be more likely to develop toxicity. CAVERJECT IMPULSE is not indicated for use in pediatric patients.
Adverse Reactions
6 ADVERSE REACTIONS
The following are discussed in more detail in other sections of the labeling:
- •
- Prolonged Erection and Priapism [see Warnings and Precautions (5.1)]
- •
- Penile Fibrosis [see Warnings and Precautions (5.2)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
CAVERJECT IMPULSE was evaluated in 87 patients in an open-label crossover study of 6 weeks treatment duration that compared the formulation of alprostadil for injection contained in CAVERJECT IMPULSE with the formulation contained in CAVERJECT. Doses used in this study ranged from 2.5 mcg to 20 mcg. Adverse reactions reported for the CAVERJECT IMPULSE formulation included: penis disorder (4.6%), prolonged erection (1.1%), injection site erythema (1.1%), rash (1.1%), dizziness (1.1%), and hematospermia (1.1%). Penis disorder included penile pain, post-injection pain, and pain with erection.
CAVERJECT IMPULSE was also evaluated in 63 patients in a single-dose, double-blind, crossover study that compared CAVERJECT IMPULSE with CAVERJECT. Doses used in this study ranged from 2.5 mcg to 20 mcg. Adverse reactions reported for the CAVERJECT IMPULSE formulation included: penile pain (1.6%) and pruritus (1.6%).
In addition to the adverse reactions observed for CAVERJECT IMPULSE in these two studies, the following adverse reactions have been reported in clinical studies of CAVERJECT:
Local Adverse Reactions: Local adverse reactions derived from 1861 patients in clinical studies of CAVERJECT, including an 18-month, open-label study, are shown in Table 1.
| |
Penile pain | 37% |
Prolonged erection | 4% |
Penile fibrosis | 3% |
Injection site hematoma | 3% |
Penis disorder* | 3% |
Injection site ecchymosis | 2% |
Penile rash | 1% |
Penile edema | 1% |
The following local adverse reactions were reported in < 1% of patients: injection site hemorrhage, injection site inflammation, injection site itching, injection site swelling, injection site edema, urethral bleeding, penile warmth, numbness, irritation, sensitivity, pruritus, erythema, painful erection, and abnormal ejaculation.
In these studies, no local adverse reactions were reported in the 294 patients who received placebo, except for penile pain (2%).
Penile Pain: In the majority of the cases, penile pain was rated mild or moderate in intensity. Three percent of patients discontinued treatment because of penile pain
Prolonged Erection/Priapism: Prolonged erection was defined as an erection that lasted for 4 to 6 hours; priapism was defined as an erection that lasted 6 hours or longer. In clinical studies, the frequency of prolonged erection after CAVERJECT was 4%, while the frequency of priapism was 0.4% [see Warnings and Precautions (5.1)].
Systemic Adverse Reactions: Systemic adverse reactions reported by ≥ 1% of subjects in clinical studies of CAVERJECT included: dizziness (1%).
The following systemic adverse reactions were reported in < 1% of patients: testicular pain, scrotal edema, hematuria, pelvic pain, hypotension, vasodilation, vasovagal reaction, diaphoresis, rash, and non-application site pruritus. Three patients (0.2%) discontinued due to symptomatic hypotension.
No systemic adverse reactions were reported in the 294 patients who received placebo.
6.2 Post-marketing Experience
The following additional adverse reactions have been reported during post approval use of CAVERJECT IMPULSE. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Injury and procedural complication: device malfunction/failure, needle breakage, drug ineffective and drug effect decreased.
Drug Interactions
7 DRUG INTERACTIONS
The potential for pharmacokinetic drug-drug interactions between alprostadil and other agents administered orally or intracavernosally has not been formally studied [see Warnings and Precautions (5.6)].
Use in Specific Populations
8 USE IN SPECIFIC POPULATIONS
8.4 Pediatric Use
CAVERJECT IMPULSE is not indicated for use in pediatric patients [see Warnings and Precautions (5.8)].
8.5 Geriatric Use
A total of 341 subjects included in clinical studies were 65 and older. No overall differences in safety and effectiveness were observed between these subjects and younger subjects, and the other reported clinical experience has not identified differences in responses between elderly and younger patients.
Overdosage
10 OVERDOSAGE
Overdosage was not observed in clinical trials with CAVERJECT IMPULSE. If intracavernous overdose of CAVERJECT IMPULSE occurs, the patient should be under medical supervision until any systemic effects have resolved and/or until penile detumescence has occurred. Treatment of any systemic symptoms (e.g., hypotension) would be appropriate.
Description
11 DESCRIPTION
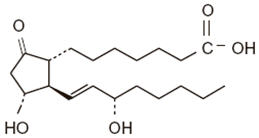
CAVERJECT IMPULSE contains alprostadil a synthetic form of prostaglandin E1 (PGE1) and is designated chemically as (11α,13E,15S)-11,15-dihydroxy-9-oxoprost-13-en-1-oic acid. The molecular weight is 354.49.
Alprostadil is a white to off-white crystalline powder with a melting point between 115° and 116°C. Its solubility at 35°C is 8000 micrograms (mcg) per 100 milliliter double distilled water.
The structural formula of alprostadil is represented below:
CAVERJECT IMPULSE is available as a disposable, single-dose, dual chamber syringe system. The system includes a glass cartridge which contains sterile, freeze-dried alprostadil in the front chamber and sterile bacteriostatic water for injection in the rear chamber. The alprostadil is reconstituted with the sterile bacteriostatic water just before injection. CAVERJECT IMPULSE is available in two strengths for intracavernosal administration:
10 microgram – The reconstituted solution has a volume of 0.64 mL. The delivered volume, 0.5 mL, contains 10 micrograms (mcg) of alprostadil, 324.7 mcg of alpha cyclodextrin, 45.4 mg of lactose, 23.5 mcg of sodium citrate, and 4.45 mg of benzyl alcohol.
20 microgram – The reconstituted solution has a volume of 0.64 mL. The delivered volume, 0.5 mL, contains 20 micrograms (mcg) of alprostadil, 649.3 mcg of alpha cyclodextrin, 45.4 mg of lactose, 23.5 mcg of sodium citrate, and 4.45 mg of benzyl alcohol.
During manufacture, the pH of the alprostadil for injection was adjusted with hydrochloric acid and/or sodium hydroxide before lyophilization.
Clinical Pharmacology
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Alprostadil induces erection by relaxation of trabecular smooth muscle and by dilation of cavernosal arteries. This leads to expansion of lacunar spaces and entrapment of blood by compressing the venules against the tunica albuginea, a process referred to as the corporal veno-occlusive mechanism.
12.3 Pharmacokinetics
Absorption: For the treatment of erectile dysfunction, alprostadil is administered by injection into the corpora cavernosa. The absolute bioavailability of alprostadil has not been determined.
Distribution: Following intracavernosal injection of 20 mcg alprostadil, mean peripheral plasma concentrations of alprostadil at 30 and 60 minutes after injection (89 and 102 picograms/mL, respectively) were not significantly greater than baseline levels of endogenous alprostadil (96 picograms/mL). Plasma levels of alprostadil were measured using a radioimmunoassay method. Alprostadil is bound in plasma primarily to albumin (81% bound) and to a lesser extent α-globulin IV-4 fraction (55% bound). No significant binding to erythrocytes or white blood cells was observed.
Metabolism: Alprostadil is converted to compounds, which are further metabolized prior to excretion. Following intravenous administration, approximately 80% of circulating alprostadil is metabolized in one pass through the lungs, primarily by beta- and omega-oxidation. Following intracavernosal injection of 20 mcg alprostadil, peripheral levels of the major circulating metabolite, 13, 14-dihydro-15-oxo-PGE1, increased to reach a peak 30 minutes after injection and returned to pre-dose levels by 60 minutes after injection.
Excretion: The metabolites of alprostadil are excreted primarily by the kidney, with almost 90% of an administered intravenous dose excreted in urine within 24 hours post-dose. The remainder of the dose is excreted in the feces. There is no evidence of tissue retention of alprostadil or its metabolites following intravenous administration.
Nonclinical Toxicology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Long-term carcinogenicity studies have not been conducted.
Mutagenesis
The following battery of mutagenicity assays revealed no potential for mutagenesis: bacterial mutation (Ames), alkaline elution, rat micronucleus, sister chromatid exchange, CHO/HGPRT mammalian cell forward gene mutation, and unscheduled DNA synthesis (UDS).
Impairment of Fertility
Rat reproductive studies indicate that alprostadil at doses of up to 0.2 mg/kg/day does not adversely affect or alter male rat fertility. These doses are approximately 32-fold higher than the maximum recommended human dose of 60 mcg based on body surface area.
Clinical Studies
14 CLINICAL STUDIES
The efficacy of CAVERJECT IMPULSE was based upon clinical studies of CAVERJECT.
The efficacy of CAVERJECT was investigated in men with a diagnosis of erectile dysfunction due to psychogenic, vasculogenic, neurogenic, and/or mixed etiology in two double-blind placebo-controlled studies (Study 1 and Study 2) and in one 6-month open-label study (Study 3). In clinical studies (Study 1 and Study 3), over 80% of patients experienced an erection sufficient for sexual intercourse after intracavernosal injection of CAVERJECT.
Study 1: A total of 153 men with ED with a mean age of 53 years (range 23–69 years) were enrolled. The study had three phases: a 2.5 week, randomized, double-blind, placebo-controlled crossover phase in which each man received in-office injections of placebo or 2.5 mcg, 5 mcg, 7.5 mcg, or 10 mcg of CAVERJECT; a 2 week, open-label, in-office dose-titration phase to identify the optimum home-use dose (the latter dose was defined as a dose inducing an erection sufficient for intercourse and lasting ≤ 60 minutes); and a 4-week open-label, at-home phase. In the double-blind placebo-controlled, crossover phase, each dose of CAVERJECT was significantly more effective than placebo by clinical evaluation ("full penile rigidity") and by RigiScan criteria (≥ 70% rigidity for at least 10 minutes); there was no response to placebo. The percentage of responders increased with increasing doses of CAVERJECT. The overall response rates in the crossover and dose-titration phases were 76% (117/153) by clinical evaluation and 51% (78/152) by RigiScan criteria. Seventy-three percent of the injections in 102 men who used CAVERJECT in the at-home phase resulted in satisfactory intercourse. Seventy-five percent of the men who used CAVERJECT in the at-home phase remained on the dose identified as optimum for them during the dose-titration phase; 17% and 8% of the men decreased or increased their dose, respectively. The mean duration of erection per injection was 70.8 minutes.
Study 2: A total of 296 men with ED with a mean age of 54 years (range 21–74 years) were enrolled in this double-blind, placebo controlled, parallel-arm design study. The men were randomly assigned to one of five groups and received either a single dose of placebo, 2.5 mcg, 5 mcg, 10 mcg, or 20 mcg of CAVERJECT. No patient responded to placebo. The differences in the response rates in both the clinical and the RigiScan evaluations between each of the doses of CAVERJECT and placebo were statistically significant. There was also a statistically significant dose-response relationship with higher clinical response rates and higher RigiScan response rates with increasing doses of CAVERJECT (with exception of the 10-mcg dose). The mean duration of erection after injection ranged from 12 minutes after the 2.5-mcg dose to 44 minutes after the 20-mcg dose and the relationship was linear (p = .025, linear regression analysis).
Study 3: The efficacy of CAVERJECT was further evaluated in a 6-month, open-label, at-home study in 683 men with ED with a mean age of 58 years (range 20–79 years). The optimum dose of CAVERJECT was established by titration in 89% of men (606/683). A total of 471/683 men (69%) completed the 6-month study. Eighty-seven percent of the 13,762 injections of CAVERJECT administered resulted in satisfactory sexual activity. The mean duration of erection was 67.5 minutes.
The formulation of alprostadil contained in CAVERJECT IMPULSE was compared to CAVERJECT in 87 men with ED in a single-blind, crossover study. The doses used by the patients in the study ranged from 2.5 mcg to 20 mcg and were the same for both formulations. The efficacy of the two formulations was shown to be comparable, as assessed by the 30-point erectile function (EF) domain score from the International Index of Erectile Function (IIEF) and by a physician-assessment score for erectile response. The mean EF domain scores for CAVERJECT and CAVERJECT IMPULSE were 26.6 (SD=5.3) and 27.6 (SD=3.8), respectively. The mean physician's assessment scores for CAVERJECT and CAVERJECT IMPULSE were 2.6 (SD=0.6) and 2.7 (SD=0.5), respectively, based on a scale of 0 (no tumescence) to 3 (full rigidity).
How Supplied/Storage and Handling
16 HOW SUPPLIED/STORAGE AND HANDLING
CAVERJECT IMPULSE is supplied as a disposable, single-dose, dual chamber syringe system. The system includes a glass cartridge, which contains sterile, freeze-dried alprostadil in the front chamber and sterile bacteriostatic water for reconstitution in the rear chamber. The syringes contain either 12.8 or 25.6 mcg of alprostadil to allow delivery of a maximum of 10 or 20 mcg/0.5mL. Store the unreconstituted product at 68°F to 77°F (20°C to 25°C); excursions permitted to 59°F to 86°F (15°C to 30°C) [see USP Controlled Room Temperature].
When reconstituted and used as directed, the deliverable amount for the 10 mcg strength is 10 mcg/0.5 mL or an increment of 10 mcg/0.5 mL: 2.5 mcg/0.125 mL, 5 mcg/0.25 mL, or 7.5 mcg/0.375 mL of alprostadil. The deliverable amount for the 20 microgram strength is 20 mcg/0.5 mL or an increment of 20 mcg/0.5 mL: 5 mcg/ 0.125 mL, 10 mcg/0.250 mL, or 15 mcg/0.375 mL of alprostadil. The reconstituted solution should be used within 24 hours when stored between 36–77°F (2°C to 25°C). Do not freeze.
CAVERJECT IMPULSE is supplied in a carton containing 2 blister trays. Each blister tray contains one dual chamber syringe system, one needle and 2 alcohol swabs. It is available in the following strengths:
10 mcg | NDC 0009-5181-01 |
20 mcg | NDC 0009-5182-01 |
Medication Guide
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use)
Dosing and Self-Administration
To ensure safe and effective use of CAVERJECT IMPULSE, instruct and train the patient in the self-injection technique before he begins treatment with CAVERJECT IMPULSE, at home. Inform the patient the initial dose administration and dose titration will take place in the health care provider’s office [see Dosage and Administration (2.1)].
Once the home dose of CAVERJECT IMPULSE has been established instruct the patient not to change the dose without consulting their health care provider.
The patient may expect an erection to occur within 5 to 20 minutes and it should last no longer than 1 hour. CAVERJECT IMPULSE should be used no more than 3 times per week, with at least 24 hours between each use.
Inform the patient that they must visit the health care provider’s office for regular check-ups for assessment of the therapeutic benefit and safety of treatment with CAVERJECT IMPULSE.
When self-administering the patient should be instructed to [see Dosage and Administration (2.3, 2.4)]:
- •
- Discard any reconstituted solution with precipitates or discoloration
- •
- Administer the injection along the dorso-lateral aspect of the proximal third of the penis
- •
- Wipe the intended injection site with an alcohol swab prior to injection
- •
- Avoid visible veins during injection
- •
- Alternate the side of the penis that is injected and the site of injection
- •
- Compress the site of injection with an alcohol swab or sterile gauze for 5 minutes
- •
- Use each CAVERJECT IMPULSE system only once and discard after use
- •
- Not use a bent needle. Do not attempt to straighten a bent needle. Remove the needle from the syringe, discard it, and attach a new, unused sterile needle to the syringe
- •
- Not re-use or share needles and to properly discard after use.
Needle Breakage
Advise patients that needle breakage has occurred during self-injection of CAVERJECT. Advise patients to insert the needle perpendicular to the long axis of the penis to avoid bending or breakage of the needle [see Warnings and Precautions (5.7) and Adverse Reactions (6.2)].
Penile Pain
Advise patients that the most frequently occurring side-effect is penile pain after injection and is usually mild to moderate in severity [see Adverse Reactions (6.1)].
Priapism
A potentially serious adverse reaction with CAVERJECT IMPULSE is priapism. Instruct the patient to seek immediate medical assistance if an erection persists for longer than 4 hours [see Warnings and Precautions (5.1)].
Penile Fibrosis
Penile fibrosis has been reported in clinical studies with CAVERJECT. Advise the patient to report any penile pain that was not present before or that increased in intensity, as well as the occurrence of nodules or hard tissue in the penis or curvature of the erect penis to his physician as soon as possible. [see Warnings and Precautions (5.2)].
Injection Site Reactions
Inform the patient that injection of CAVERJECT IMPULSE can induce a small amount of bleeding at the site of injection and that hematoma and ecchymosis may occur. Advise the patient to report any persistent redness, tenderness or swelling [see Warnings and Precautions (5.4)].
Sexually Transmitted Disease
Use of CAVERJECT IMPULSE offers no protection from the transmission of sexually transmitted diseases. Advise the patient about the protective measures that are necessary to guard against the spread of sexually transmitted diseases, including the human immunodeficiency virus (HIV) [see Warnings and Precautions (5.9)].
Patient Information
CAVERJECT IMPULSE [KAV-er-jeckt]
(alprostadil)
for injection, for intracavernosal use
Read this Patient Information before you start using CAVERJECT IMPULSE and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or your treatment.
What is CAVERJECT IMPULSE?
CAVERJECT IMPULSE is a prescription medicine used:
- •
- to treat erectile dysfunction (ED).
- •
- with other medical tests to diagnose ED.
CAVERJECT IMPULSE is not meant for use in women or children under 18 years of age.
Do not use CAVERJECT IMPULSE if you:
- •
- are allergic to alprostadil or any of the ingredients in CAVERJECT. See the end of this leaflet for a complete list of ingredients in CAVERJECT.
- •
- have certain medical problems that might cause you to have an erection that lasts for more than 4 hours, such as sickle cell anemia, sickle cell trait, multiple myeloma, leukemia.
- •
- have a deformed penis shape
- •
- have a penile implant
Before you use CAVERJECT IMPULSE, tell your healthcare provider if you:
- •
- have had an erection that lasted more than 4 hours
- •
- have sickle cell trait or sickle cell anemia
- •
- have or have had a blood cell cancer called multiple myeloma or leukemia
- •
- have a deformed penis shape
- •
- have a penile implant
- •
- have low blood pressure (hypotension)
- •
- have bleeding problems
- •
- have or have had heart problems such as a heart attack, irregular heartbeat, angina, chest pain, narrowing of the aortic valve or heart failure
- •
- have any other medical conditions
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
CAVERJECT IMPULSE may affect the way other medicines work, and other medicines may affect the way CAVERJECT IMPULSE works causing side effects.
Especially tell your healthcare provider if you take any other medicines that are injected into your penis (intracavernosally) or certain medicines called anticoagulant medicines (such as heparin or warfarin).
Ask your healthcare provider or pharmacist for a list of these medicines, if you are not sure.
Know the medicines you take. Keep a list of them to show to your healthcare provider and pharmacist when you get a new medicine.
How should I use CAVERJECT IMPULSE?
- •
- See the detailed Instructions for Use that comes with your CAVERJECT IMPULSE for information about how to prepare and inject CAVERJECT IMPULSE the right way.
- •
- Use CAVERJECT IMPULSE exactly as your healthcare provider tells you to.
- •
- Your healthcare provider will tell you how much CAVERJECT IMPULSE to use and when to use it.
- •
- Your healthcare provider may change your dose if needed. Do not change your dose of CAVERJECT IMPULSE without first talking to your healthcare provider.
- •
- Your healthcare provider should show you how to prepare and inject CAVERJECT IMPULSE the right way before you inject it for the first time.
- •
- CAVERJECT IMPULSE should not be used more than 3 times per week.
- •
- CAVERJECT IMPULSE should not be used more than 1 time every 24 hours.
- •
- Change the exact place and side of the penis that you inject Caverject Impulse each time you use it.
- •
- CAVERJECT IMPULSE is for one use only and should be thrown away properly after a single use.
You should see your healthcare provider every 3 months for check-ups to be sure that CAVERJECT IMPULSE is working the right way and to change your CAVERJECT IMPULSE dose if needed.
What are the possible side effects of CAVERJECT IMPULSE?
CAVERJECT IMPULSE may cause serious side effects, including:
- •
- an erection that will not go away. If you have an erection that lasts more than 4 hours, get medical help right away. If it is not treated right away, this condition can permanently damage your penis.
- •
- deformed penis shape (penile fibrosis). Your healthcare provider should check your penis regularly for signs of penile fibrosis. You should not continue to use CAVERJECT IMPULSE if you get penile fibrosis.
- •
- low blood pressure (hypotension)
- •
- injection site bleeding. People who take certain medicines called anticoagulants (such as heparin or warfarin) may have a risk for increased bleeding at the injection site.
- •
- increased risk of heart problems. Sexual activity can put an extra strain on your heart, especially if your heart is weak from a heart attack or heart disease. Ask your healthcare provider if your heart is healthy enough to handle the extra strain of having sex. Stop sexual activity and get medical help right away if you get symptoms of a heart problem such as chest pain, dizziness or nausea.
- •
- needle breakage. There is a possibility of needle breakage with use of CAVERJECT IMPULSE. To best avoid breaking the needle, you should pay careful attention to your healthcare provider's instructions and handle the syringe and needle properly. If the needle is bent at any time, do not attempt to straighten it and do not use it. A bent and re-straightened needle is more likely to break. Remove the needle from the syringe, discard, and attach a new, unused sterile needle to the syringe. If the needle breaks during injection and you are able to see and grasp the broken end, you should remove it and contact your healthcare provider. If you cannot see or cannot grasp the broken end, you should promptly contact your healthcare provider.
- •
- benzyl alcohol toxicity. Benzyl alcohol is a preservative in CAVERJECT IMPULSE. Benzyl alcohol has caused serious side effects, including death, in children, especially premature and low-birth weight infants, who have received the preservative benzyl alcohol. CAVERJECT IMPULSE is not meant for use in children.
CAVERJECT IMPULSE does not protect you or your partner from getting sexually transmitted infections, including HIV-the virus that causes AIDS.
The most common side effects of CAVERJECT IMPULSE include:
- •
- penile pain
These are not all the possible side effects of CAVERJECT IMPULSE.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of CAVERJECT IMPULSE
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information Leaflet. Do not use CAVERJECT IMPULSE for a condition for which it was not prescribed. Do not give CAVERJECT IMPULSE to other people even if they have the same symptoms that you have. It may harm them.
You can ask your pharmacist or healthcare provider for information about CAVERJECT IMPULSE that is written for health professionals.
What are the Ingredients in CAVERJECT IMPULSE?
Active ingredient: alprostadil
Inactive ingredients: alpha cyclodextrin, lactose, sodium citrate, benzyl alcohol and hydrochloric and/or sodium hydroxide for pH adjustment.
This product’s label may have been updated. For current full prescribing information, please visit www.pfizer.com
LAB-0641-8.0
Revised January 2023
Instructions For Use
INSTRUCTIONS FOR USE
CAVERJECT [KAV-er-jeckt]®
(alprostadil)
for injection, for intracavernosal use
Before you use CAVERJECT, your doctor must train you in how to prepare and give the injection properly.
Before using CAVERJECT, talk to your doctor about what to expect when using it, possible side effects, and what to do if side effects occur. Your dose has been selected for your individual needs. Do not change your dose without consulting your doctor. If you are not sure of the volume or dose to be used, talk to your doctor or pharmacist.
Follow these instructions exactly to properly prepare the syringe for use, and to correctly inject a sterile (germ-free) dose of CAVERJECT.
Supplies Needed
The CAVERJECT IMPULSE carton contains two (2) sealed plastic trays, with one dose of Caverject in each tray.
Each tray contains: (a) a syringe, (b) a separate needle assembly and (c) two alcohol swabs. The syringe and the needle assembly are shown in Figure A (below). Please note that the needle assembly is packaged as a single piece and is sealed with a paper cover on the bottom.
DO NOT try to assemble the syringe until you have read ALL of the instructions.
First-read through ALL of the instructions (Step 1 through Step 12) before trying to assemble the syringe. Then go back to Step 1 and begin to prepare the syringe for use.
CAVERJECT IMPULSE is available in two versions: the 10 mcg strength (white plunger) and the 20 mcg strength (blue plunger). Each syringe is designed to be used only one time, but you can select the dose that will be delivered:
- •
- The 10 mcg strength syringe (white plunger) can deliver 10 mcg (the full dose), or one of three partial doses: 7.5 mcg, or 5 mcg, or 2.5 mcg.
- •
- The 20 mcg strength syringe (blue plunger) can deliver 20 mcg (the full dose), or one of three partial doses: 15 mcg, or 10 mcg, or 5 mcg.
If you deliver a partial dose there will be left-over solution in the syringe – this is normal.
MAKE SURE YOU HAVE THE CORRECT STRENGTH OF CAVERJECT IMPULSE to deliver your assigned dose
INSTRUCTIONS for PREPARING the SYRINGE (Step 1 through Step 12)
Wash your hands thoroughly and dry them with a clean towel. | |
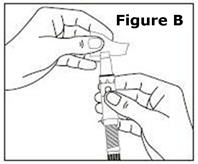
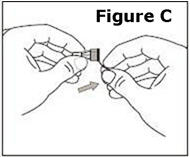
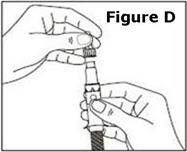
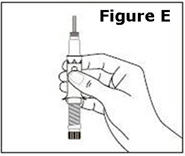
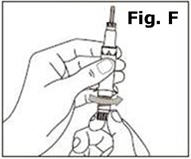
STEP 1. | Open the sealed plastic tray. Remove the syringe, the needle assembly, and the alcohol swabs from the tray. All items should be present. |
Look at the needle assembly. The needle assembly is a sealed unit that contains the outer protective cap, the inner protective cap, and the superfine needle, as shown in Figure A. It is sealed with a small round paper cover (not shown in Figure A). | |
Do not open the needle assembly at this point – leave it sealed inside the outer protective cap. | |
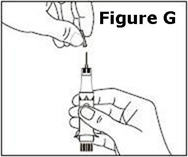
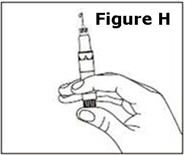
Next, examine the syringe. Find the location of the dose window. Right now you will not see anything in this window, but at a later Step, a number will appear in this window (the dose to be delivered). | |
Finally, look at the Plunger, but do not move it at this time. During the assembly process, some Steps may ask you to ROTATE the plunger and other steps may ask you to PUSH the plunger. | |
It is important to only rotate the plunger – or only push – as directed in each Step, but DO NOT do both at the same time. |
|
 |
|
| |
 |
|
| |
 |
|
| |
 |
|
| |
 |
|
| |
 |
| ||
 |
| ||
|
| ||
 |
|
| |
 |
|
| |
 |
| ||
 |
| ||
 |
| ||
 |
| ||
| |||
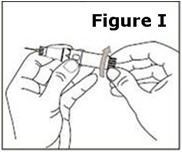
STEP 12. | The syringe is now ready for use. |
How to give the injection
- •
- Make yourself comfortable. Take some time to relax yourself and your partner. If your doctor has recommended you use an alcohol cleansing swab, open one now.
- •
- Make sure that the needle is not bent. If it is, do not use it. Do not attempt to straighten the needle out. Properly discard it.
 |
|
 | |
 |
|
Some liquid will remain in the syringe if you delivered a PARTIAL dose. This is expected. Do not try to inject left-over liquid from a partial dose. Syringes used to inject a partial dose must be discarded since each syringe can only be used one time. Discard syringes with left-over liquid.
- o
- A partial dose is 5 mcg, 10 mcg or 15 mcg for the BLUE plunger syringe.
- o
- A partial dose is 2.5 mcg, 5 mcg, or 7.5 mcg for the WHITE plunger syringe.
Do not keep any solution in the cartridge to use for a second injection. When you have finished with the syringe, discard it carefully as recommended by your doctor, so no one will use it or stick themselves with it.
After your injection:
Dispose of your used CAVERJECT IMPULSE syringes and needle.
- •
- Put your used CAVERJECT IMPULSE syringe and needle in a FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) loose needles and syringes in your household trash.
- •
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- o
- made of a heavy-duty plastic
- o
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out
- o
- upright and stable during use
- o
- leak-resistant
- o
- properly labeled to warn of hazardous waste inside the container
- •
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. Do not reuse or share your needles or syringes with other people. For more information about the safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at: http://www.fda.gov/safesharpsdisposal.
- •
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
How should I store CAVERJECT IMPULSE?
- 1.
- Store unmixed CAVERJECT IMPULSE at room temperature between 68°F to 77°F (20°C to 25°C).
- 2.
- Store mixed CAVERJECT IMPULSE between 36°F to 77°F (2°C to 25°C). Do not freeze.
- 3.
- CAVERJECT IMPULSE should be used within 24 hours after it is mixed.
Keep CAVERJECT IMPULSE and all medicines out of the reach of children.
This Patient Information and Instructions for Use has been approved by the U.S Food and Drug Administration.
LAB-1504-2.0
Revised January 2023
Other
This product's label may have been updated. For current full prescribing information, please visit www.pfizer.com
LAB-0007-10.0
Patient Information
Patient Information
CAVERJECT IMPULSE [KAV-er-jeckt]
(alprostadil)
for injection, for intracavernosal use
Read this Patient Information before you start using CAVERJECT IMPULSE and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or your treatment.
What is CAVERJECT IMPULSE?
What is CAVERJECT IMPULSE?
CAVERJECT IMPULSE is a prescription medicine used:
- •
- to treat erectile dysfunction (ED).
- •
- with other medical tests to diagnose ED.
CAVERJECT IMPULSE is not meant for use in women or children under 18 years of age.
Do not use CAVERJECT IMPULSE if you:
- •
- are allergic to alprostadil or any of the ingredients in CAVERJECT. See the end of this leaflet for a complete list of ingredients in CAVERJECT.
- •
- have certain medical problems that might cause you to have an erection that lasts for more than 4 hours, such as sickle cell anemia, sickle cell trait, multiple myeloma, leukemia.
- •
- have a deformed penis shape
- •
- have a penile implant
Before you use CAVERJECT IMPULSE, tell your healthcare provider if you:
- •
- have had an erection that lasted more than 4 hours
- •
- have sickle cell trait or sickle cell anemia
- •
- have or have had a blood cell cancer called multiple myeloma or leukemia
- •
- have a deformed penis shape
- •
- have a penile implant
- •
- have low blood pressure (hypotension)
- •
- have bleeding problems
- •
- have or have had heart problems such as a heart attack, irregular heartbeat, angina, chest pain, narrowing of the aortic valve or heart failure
- •
- have any other medical conditions
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
CAVERJECT IMPULSE may affect the way other medicines work, and other medicines may affect the way CAVERJECT IMPULSE works causing side effects.
Especially tell your healthcare provider if you take any other medicines that are injected into your penis (intracavernosally) or certain medicines called anticoagulant medicines (such as heparin or warfarin).
Ask your healthcare provider or pharmacist for a list of these medicines, if you are not sure.
Know the medicines you take. Keep a list of them to show to your healthcare provider and pharmacist when you get a new medicine.
How should I use CAVERJECT IMPULSE?
How should I use CAVERJECT IMPULSE?
- •
- See the detailed Instructions for Use that comes with your CAVERJECT IMPULSE for information about how to prepare and inject CAVERJECT IMPULSE the right way.
- •
- Use CAVERJECT IMPULSE exactly as your healthcare provider tells you to.
- •
- Your healthcare provider will tell you how much CAVERJECT IMPULSE to use and when to use it.
- •
- Your healthcare provider may change your dose if needed. Do not change your dose of CAVERJECT IMPULSE without first talking to your healthcare provider.
- •
- Your healthcare provider should show you how to prepare and inject CAVERJECT IMPULSE the right way before you inject it for the first time.
- •
- CAVERJECT IMPULSE should not be used more than 3 times per week.
- •
- CAVERJECT IMPULSE should not be used more than 1 time every 24 hours.
- •
- Change the exact place and side of the penis that you inject Caverject Impulse each time you use it.
- •
- CAVERJECT IMPULSE is for one use only and should be thrown away properly after a single use.
You should see your healthcare provider every 3 months for check-ups to be sure that CAVERJECT IMPULSE is working the right way and to change your CAVERJECT IMPULSE dose if needed.
What are the possible side effects of CAVERJECT IMPULSE?
What are the possible side effects of CAVERJECT IMPULSE?
CAVERJECT IMPULSE may cause serious side effects, including:
- •
- an erection that will not go away. If you have an erection that lasts more than 4 hours, get medical help right away. If it is not treated right away, this condition can permanently damage your penis.
- •
- deformed penis shape (penile fibrosis). Your healthcare provider should check your penis regularly for signs of penile fibrosis. You should not continue to use CAVERJECT IMPULSE if you get penile fibrosis.
- •
- low blood pressure (hypotension)
- •
- injection site bleeding. People who take certain medicines called anticoagulants (such as heparin or warfarin) may have a risk for increased bleeding at the injection site.
- •
- increased risk of heart problems. Sexual activity can put an extra strain on your heart, especially if your heart is weak from a heart attack or heart disease. Ask your healthcare provider if your heart is healthy enough to handle the extra strain of having sex. Stop sexual activity and get medical help right away if you get symptoms of a heart problem such as chest pain, dizziness or nausea.
- •
- needle breakage. There is a possibility of needle breakage with use of CAVERJECT IMPULSE. To best avoid breaking the needle, you should pay careful attention to your healthcare provider's instructions and handle the syringe and needle properly. If the needle is bent at any time, do not attempt to straighten it and do not use it. A bent and re-straightened needle is more likely to break. Remove the needle from the syringe, discard, and attach a new, unused sterile needle to the syringe. If the needle breaks during injection and you are able to see and grasp the broken end, you should remove it and contact your healthcare provider. If you cannot see or cannot grasp the broken end, you should promptly contact your healthcare provider.
- •
- benzyl alcohol toxicity. Benzyl alcohol is a preservative in CAVERJECT IMPULSE. Benzyl alcohol has caused serious side effects, including death, in children, especially premature and low-birth weight infants, who have received the preservative benzyl alcohol. CAVERJECT IMPULSE is not meant for use in children.
CAVERJECT IMPULSE does not protect you or your partner from getting sexually transmitted infections, including HIV-the virus that causes AIDS.
The most common side effects of CAVERJECT IMPULSE include:
- •
- penile pain
These are not all the possible side effects of CAVERJECT IMPULSE.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of CAVERJECT IMPULSE
General information about the safe and effective use of CAVERJECT IMPULSE
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information Leaflet. Do not use CAVERJECT IMPULSE for a condition for which it was not prescribed. Do not give CAVERJECT IMPULSE to other people even if they have the same symptoms that you have. It may harm them.
You can ask your pharmacist or healthcare provider for information about CAVERJECT IMPULSE that is written for health professionals.
What are the Ingredients in CAVERJECT IMPULSE?
What are the Ingredients in CAVERJECT IMPULSE?
Active ingredient: alprostadil
Inactive ingredients: alpha cyclodextrin, lactose, sodium citrate, benzyl alcohol and hydrochloric and/or sodium hydroxide for pH adjustment.
This product’s label may have been updated. For current full prescribing information, please visit www.pfizer.com
LAB-0641-8.0
Revised January 2023
INSTRUCTIONS FOR USECAVERJECT [KAV-er-jeckt]®(alprostadil)for injection, for intracavernosal use
INSTRUCTIONS FOR USECAVERJECT [KAV-er-jeckt]®(alprostadil)for injection, for intracavernosal use
INSTRUCTIONS FOR USEBefore you use CAVERJECT, your doctor must train you in how to prepare and give the injection properly.
Before using CAVERJECT, talk to your doctor about what to expect when using it, possible side effects, and what to do if side effects occur. Your dose has been selected for your individual needs. Do not change your dose without consulting your doctor. If you are not sure of the volume or dose to be used, talk to your doctor or pharmacist.
Follow these instructions exactly to properly prepare the syringe for use, and to correctly inject a sterile (germ-free) dose of CAVERJECT.
Supplies Needed
The CAVERJECT IMPULSE carton contains two (2) sealed plastic trays, with one dose of Caverject in each tray.
Each tray contains: (a) a syringe, (b) a separate needle assembly and (c) two alcohol swabs. The syringe and the needle assembly are shown in Figure A (below). Please note that the needle assembly is packaged as a single piece and is sealed with a paper cover on the bottom.
DO NOT try to assemble the syringe until you have read ALL of the instructions.
First-read through ALL of the instructions (Step 1 through Step 12) before trying to assemble the syringe. Then go back to Step 1 and begin to prepare the syringe for use.
CAVERJECT IMPULSE is available in two versions: the 10 mcg strength (white plunger) and the 20 mcg strength (blue plunger). Each syringe is designed to be used only one time, but you can select the dose that will be delivered:
- •
- The 10 mcg strength syringe (white plunger) can deliver 10 mcg (the full dose), or one of three partial doses: 7.5 mcg, or 5 mcg, or 2.5 mcg.
- •
- The 20 mcg strength syringe (blue plunger) can deliver 20 mcg (the full dose), or one of three partial doses: 15 mcg, or 10 mcg, or 5 mcg.
If you deliver a partial dose there will be left-over solution in the syringe – this is normal.
MAKE SURE YOU HAVE THE CORRECT STRENGTH OF CAVERJECT IMPULSE to deliver your assigned dose
INSTRUCTIONS for PREPARING the SYRINGE (Step 1 through Step 12)
Wash your hands thoroughly and dry them with a clean towel. | |
STEP 1. | Open the sealed plastic tray. Remove the syringe, the needle assembly, and the alcohol swabs from the tray. All items should be present. |
Look at the needle assembly. The needle assembly is a sealed unit that contains the outer protective cap, the inner protective cap, and the superfine needle, as shown in Figure A. It is sealed with a small round paper cover (not shown in Figure A). | |
Do not open the needle assembly at this point – leave it sealed inside the outer protective cap. | |
Next, examine the syringe. Find the location of the dose window. Right now you will not see anything in this window, but at a later Step, a number will appear in this window (the dose to be delivered). | |
Finally, look at the Plunger, but do not move it at this time. During the assembly process, some Steps may ask you to ROTATE the plunger and other steps may ask you to PUSH the plunger. | |
It is important to only rotate the plunger – or only push – as directed in each Step, but DO NOT do both at the same time. |
|
 |
|
| |
 |
|
| |
 |
|
| |
 |
|
| |
 |
|
| |
 |
| ||
 |
| ||
|
| ||
 |
|
| |
 |
|
| |
 |
| ||
 |
| ||
 |
| ||
 |
| ||
| |||
STEP 12. | The syringe is now ready for use. |
How to give the injection
- •
- Make yourself comfortable. Take some time to relax yourself and your partner. If your doctor has recommended you use an alcohol cleansing swab, open one now.
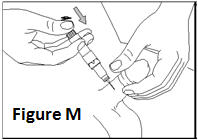
- •
- Make sure that the needle is not bent. If it is, do not use it. Do not attempt to straighten the needle out. Properly discard it.
 |
|
 | |
 |
|
Some liquid will remain in the syringe if you delivered a PARTIAL dose. This is expected. Do not try to inject left-over liquid from a partial dose. Syringes used to inject a partial dose must be discarded since each syringe can only be used one time. Discard syringes with left-over liquid.
- o
- A partial dose is 5 mcg, 10 mcg or 15 mcg for the BLUE plunger syringe.
- o
- A partial dose is 2.5 mcg, 5 mcg, or 7.5 mcg for the WHITE plunger syringe.
Do not keep any solution in the cartridge to use for a second injection. When you have finished with the syringe, discard it carefully as recommended by your doctor, so no one will use it or stick themselves with it.
After your injection:
Dispose of your used CAVERJECT IMPULSE syringes and needle.
- •
- Put your used CAVERJECT IMPULSE syringe and needle in a FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) loose needles and syringes in your household trash.
- •
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- o
- made of a heavy-duty plastic
- o
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out
- o
- upright and stable during use
- o
- leak-resistant
- o
- properly labeled to warn of hazardous waste inside the container
- •
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. Do not reuse or share your needles or syringes with other people. For more information about the safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at: http://www.fda.gov/safesharpsdisposal.
- •
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
How should I store CAVERJECT IMPULSE?
How should I store CAVERJECT IMPULSE?
- 1.
- Store unmixed CAVERJECT IMPULSE at room temperature between 68°F to 77°F (20°C to 25°C).
- 2.
- Store mixed CAVERJECT IMPULSE between 36°F to 77°F (2°C to 25°C). Do not freeze.
- 3.
- CAVERJECT IMPULSE should be used within 24 hours after it is mixed.
Keep CAVERJECT IMPULSE and all medicines out of the reach of children.
This Patient Information and Instructions for Use has been approved by the U.S Food and Drug Administration.
LAB-1504-2.0
Revised January 2023
Full Patient Information
Full Patient Information
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use)
Dosing and Self-Administration
To ensure safe and effective use of CAVERJECT IMPULSE, instruct and train the patient in the self-injection technique before he begins treatment with CAVERJECT IMPULSE, at home. Inform the patient the initial dose administration and dose titration will take place in the health care provider’s office [see Dosage and Administration (2.1)].
Once the home dose of CAVERJECT IMPULSE has been established instruct the patient not to change the dose without consulting their health care provider.
The patient may expect an erection to occur within 5 to 20 minutes and it should last no longer than 1 hour. CAVERJECT IMPULSE should be used no more than 3 times per week, with at least 24 hours between each use.
Inform the patient that they must visit the health care provider’s office for regular check-ups for assessment of the therapeutic benefit and safety of treatment with CAVERJECT IMPULSE.
When self-administering the patient should be instructed to [see Dosage and Administration (2.3, 2.4)]:
- •
- Discard any reconstituted solution with precipitates or discoloration
- •
- Administer the injection along the dorso-lateral aspect of the proximal third of the penis
- •
- Wipe the intended injection site with an alcohol swab prior to injection
- •
- Avoid visible veins during injection
- •
- Alternate the side of the penis that is injected and the site of injection
- •
- Compress the site of injection with an alcohol swab or sterile gauze for 5 minutes
- •
- Use each CAVERJECT IMPULSE system only once and discard after use
- •
- Not use a bent needle. Do not attempt to straighten a bent needle. Remove the needle from the syringe, discard it, and attach a new, unused sterile needle to the syringe
- •
- Not re-use or share needles and to properly discard after use.
Needle Breakage
Advise patients that needle breakage has occurred during self-injection of CAVERJECT. Advise patients to insert the needle perpendicular to the long axis of the penis to avoid bending or breakage of the needle [see Warnings and Precautions (5.7) and Adverse Reactions (6.2)].
Penile Pain
Advise patients that the most frequently occurring side-effect is penile pain after injection and is usually mild to moderate in severity [see Adverse Reactions (6.1)].
Priapism
A potentially serious adverse reaction with CAVERJECT IMPULSE is priapism. Instruct the patient to seek immediate medical assistance if an erection persists for longer than 4 hours [see Warnings and Precautions (5.1)].
Penile Fibrosis
Penile fibrosis has been reported in clinical studies with CAVERJECT. Advise the patient to report any penile pain that was not present before or that increased in intensity, as well as the occurrence of nodules or hard tissue in the penis or curvature of the erect penis to his physician as soon as possible. [see Warnings and Precautions (5.2)].
Injection Site Reactions
Inform the patient that injection of CAVERJECT IMPULSE can induce a small amount of bleeding at the site of injection and that hematoma and ecchymosis may occur. Advise the patient to report any persistent redness, tenderness or swelling [see Warnings and Precautions (5.4)].
Sexually Transmitted Disease
Use of CAVERJECT IMPULSE offers no protection from the transmission of sexually transmitted diseases. Advise the patient about the protective measures that are necessary to guard against the spread of sexually transmitted diseases, including the human immunodeficiency virus (HIV) [see Warnings and Precautions (5.9)].
Patient Information
CAVERJECT IMPULSE [KAV-er-jeckt]
(alprostadil)
for injection, for intracavernosal use
Read this Patient Information before you start using CAVERJECT IMPULSE and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or your treatment.
What is CAVERJECT IMPULSE?
CAVERJECT IMPULSE is a prescription medicine used:
- •
- to treat erectile dysfunction (ED).
- •
- with other medical tests to diagnose ED.
CAVERJECT IMPULSE is not meant for use in women or children under 18 years of age.
Do not use CAVERJECT IMPULSE if you:
- •
- are allergic to alprostadil or any of the ingredients in CAVERJECT. See the end of this leaflet for a complete list of ingredients in CAVERJECT.
- •
- have certain medical problems that might cause you to have an erection that lasts for more than 4 hours, such as sickle cell anemia, sickle cell trait, multiple myeloma, leukemia.
- •
- have a deformed penis shape
- •
- have a penile implant
Before you use CAVERJECT IMPULSE, tell your healthcare provider if you:
- •
- have had an erection that lasted more than 4 hours
- •
- have sickle cell trait or sickle cell anemia
- •
- have or have had a blood cell cancer called multiple myeloma or leukemia
- •
- have a deformed penis shape
- •
- have a penile implant
- •
- have low blood pressure (hypotension)
- •
- have bleeding problems
- •
- have or have had heart problems such as a heart attack, irregular heartbeat, angina, chest pain, narrowing of the aortic valve or heart failure
- •
- have any other medical conditions
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
CAVERJECT IMPULSE may affect the way other medicines work, and other medicines may affect the way CAVERJECT IMPULSE works causing side effects.
Especially tell your healthcare provider if you take any other medicines that are injected into your penis (intracavernosally) or certain medicines called anticoagulant medicines (such as heparin or warfarin).
Ask your healthcare provider or pharmacist for a list of these medicines, if you are not sure.
Know the medicines you take. Keep a list of them to show to your healthcare provider and pharmacist when you get a new medicine.
How should I use CAVERJECT IMPULSE?
- •
- See the detailed Instructions for Use that comes with your CAVERJECT IMPULSE for information about how to prepare and inject CAVERJECT IMPULSE the right way.
- •
- Use CAVERJECT IMPULSE exactly as your healthcare provider tells you to.
- •
- Your healthcare provider will tell you how much CAVERJECT IMPULSE to use and when to use it.
- •
- Your healthcare provider may change your dose if needed. Do not change your dose of CAVERJECT IMPULSE without first talking to your healthcare provider.
- •
- Your healthcare provider should show you how to prepare and inject CAVERJECT IMPULSE the right way before you inject it for the first time.
- •
- CAVERJECT IMPULSE should not be used more than 3 times per week.
- •
- CAVERJECT IMPULSE should not be used more than 1 time every 24 hours.
- •
- Change the exact place and side of the penis that you inject Caverject Impulse each time you use it.
- •
- CAVERJECT IMPULSE is for one use only and should be thrown away properly after a single use.
You should see your healthcare provider every 3 months for check-ups to be sure that CAVERJECT IMPULSE is working the right way and to change your CAVERJECT IMPULSE dose if needed.
What are the possible side effects of CAVERJECT IMPULSE?
CAVERJECT IMPULSE may cause serious side effects, including:
- •
- an erection that will not go away. If you have an erection that lasts more than 4 hours, get medical help right away. If it is not treated right away, this condition can permanently damage your penis.
- •
- deformed penis shape (penile fibrosis). Your healthcare provider should check your penis regularly for signs of penile fibrosis. You should not continue to use CAVERJECT IMPULSE if you get penile fibrosis.
- •
- low blood pressure (hypotension)
- •
- injection site bleeding. People who take certain medicines called anticoagulants (such as heparin or warfarin) may have a risk for increased bleeding at the injection site.
- •
- increased risk of heart problems. Sexual activity can put an extra strain on your heart, especially if your heart is weak from a heart attack or heart disease. Ask your healthcare provider if your heart is healthy enough to handle the extra strain of having sex. Stop sexual activity and get medical help right away if you get symptoms of a heart problem such as chest pain, dizziness or nausea.
- •
- needle breakage. There is a possibility of needle breakage with use of CAVERJECT IMPULSE. To best avoid breaking the needle, you should pay careful attention to your healthcare provider's instructions and handle the syringe and needle properly. If the needle is bent at any time, do not attempt to straighten it and do not use it. A bent and re-straightened needle is more likely to break. Remove the needle from the syringe, discard, and attach a new, unused sterile needle to the syringe. If the needle breaks during injection and you are able to see and grasp the broken end, you should remove it and contact your healthcare provider. If you cannot see or cannot grasp the broken end, you should promptly contact your healthcare provider.
- •
- benzyl alcohol toxicity. Benzyl alcohol is a preservative in CAVERJECT IMPULSE. Benzyl alcohol has caused serious side effects, including death, in children, especially premature and low-birth weight infants, who have received the preservative benzyl alcohol. CAVERJECT IMPULSE is not meant for use in children.
CAVERJECT IMPULSE does not protect you or your partner from getting sexually transmitted infections, including HIV-the virus that causes AIDS.
The most common side effects of CAVERJECT IMPULSE include:
- •
- penile pain
These are not all the possible side effects of CAVERJECT IMPULSE.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of CAVERJECT IMPULSE
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information Leaflet. Do not use CAVERJECT IMPULSE for a condition for which it was not prescribed. Do not give CAVERJECT IMPULSE to other people even if they have the same symptoms that you have. It may harm them.
You can ask your pharmacist or healthcare provider for information about CAVERJECT IMPULSE that is written for health professionals.
What are the Ingredients in CAVERJECT IMPULSE?
Active ingredient: alprostadil
Inactive ingredients: alpha cyclodextrin, lactose, sodium citrate, benzyl alcohol and hydrochloric and/or sodium hydroxide for pH adjustment.
This product’s label may have been updated. For current full prescribing information, please visit www.pfizer.com
LAB-0641-8.0
Revised January 2023
INSTRUCTIONS FOR USE
CAVERJECT [KAV-er-jeckt]®
(alprostadil)
for injection, for intracavernosal use
Before you use CAVERJECT, your doctor must train you in how to prepare and give the injection properly.
Before using CAVERJECT, talk to your doctor about what to expect when using it, possible side effects, and what to do if side effects occur. Your dose has been selected for your individual needs. Do not change your dose without consulting your doctor. If you are not sure of the volume or dose to be used, talk to your doctor or pharmacist.
Follow these instructions exactly to properly prepare the syringe for use, and to correctly inject a sterile (germ-free) dose of CAVERJECT.
Supplies Needed
The CAVERJECT IMPULSE carton contains two (2) sealed plastic trays, with one dose of Caverject in each tray.
Each tray contains: (a) a syringe, (b) a separate needle assembly and (c) two alcohol swabs. The syringe and the needle assembly are shown in Figure A (below). Please note that the needle assembly is packaged as a single piece and is sealed with a paper cover on the bottom.
DO NOT try to assemble the syringe until you have read ALL of the instructions.
First-read through ALL of the instructions (Step 1 through Step 12) before trying to assemble the syringe. Then go back to Step 1 and begin to prepare the syringe for use.
CAVERJECT IMPULSE is available in two versions: the 10 mcg strength (white plunger) and the 20 mcg strength (blue plunger). Each syringe is designed to be used only one time, but you can select the dose that will be delivered:
- •
- The 10 mcg strength syringe (white plunger) can deliver 10 mcg (the full dose), or one of three partial doses: 7.5 mcg, or 5 mcg, or 2.5 mcg.
- •
- The 20 mcg strength syringe (blue plunger) can deliver 20 mcg (the full dose), or one of three partial doses: 15 mcg, or 10 mcg, or 5 mcg.
If you deliver a partial dose there will be left-over solution in the syringe – this is normal.
MAKE SURE YOU HAVE THE CORRECT STRENGTH OF CAVERJECT IMPULSE to deliver your assigned dose
INSTRUCTIONS for PREPARING the SYRINGE (Step 1 through Step 12)
Wash your hands thoroughly and dry them with a clean towel. | |
STEP 1. | Open the sealed plastic tray. Remove the syringe, the needle assembly, and the alcohol swabs from the tray. All items should be present. |
Look at the needle assembly. The needle assembly is a sealed unit that contains the outer protective cap, the inner protective cap, and the superfine needle, as shown in Figure A. It is sealed with a small round paper cover (not shown in Figure A). | |
Do not open the needle assembly at this point – leave it sealed inside the outer protective cap. | |
Next, examine the syringe. Find the location of the dose window. Right now you will not see anything in this window, but at a later Step, a number will appear in this window (the dose to be delivered). | |
Finally, look at the Plunger, but do not move it at this time. During the assembly process, some Steps may ask you to ROTATE the plunger and other steps may ask you to PUSH the plunger. | |
It is important to only rotate the plunger – or only push – as directed in each Step, but DO NOT do both at the same time. |
|
 |
|
| |
 |
|
| |
 |
|
| |
 |
|
| |
 |
|
| |
 |
| ||
 |
| ||
|
| ||
 |
|
| |
 |
|
| |
 |
| ||
 |
| ||
 |
| ||
 |
| ||
| |||
STEP 12. | The syringe is now ready for use. |
How to give the injection
- •
- Make yourself comfortable. Take some time to relax yourself and your partner. If your doctor has recommended you use an alcohol cleansing swab, open one now.
- •
- Make sure that the needle is not bent. If it is, do not use it. Do not attempt to straighten the needle out. Properly discard it.
 |
|
 | |
 |
|
Some liquid will remain in the syringe if you delivered a PARTIAL dose. This is expected. Do not try to inject left-over liquid from a partial dose. Syringes used to inject a partial dose must be discarded since each syringe can only be used one time. Discard syringes with left-over liquid.
- o
- A partial dose is 5 mcg, 10 mcg or 15 mcg for the BLUE plunger syringe.
- o
- A partial dose is 2.5 mcg, 5 mcg, or 7.5 mcg for the WHITE plunger syringe.
Do not keep any solution in the cartridge to use for a second injection. When you have finished with the syringe, discard it carefully as recommended by your doctor, so no one will use it or stick themselves with it.
After your injection:
Dispose of your used CAVERJECT IMPULSE syringes and needle.
- •
- Put your used CAVERJECT IMPULSE syringe and needle in a FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) loose needles and syringes in your household trash.
- •
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- o
- made of a heavy-duty plastic
- o
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out
- o
- upright and stable during use
- o
- leak-resistant
- o
- properly labeled to warn of hazardous waste inside the container
- •
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. Do not reuse or share your needles or syringes with other people. For more information about the safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at: http://www.fda.gov/safesharpsdisposal.
- •
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
How should I store CAVERJECT IMPULSE?
- 1.
- Store unmixed CAVERJECT IMPULSE at room temperature between 68°F to 77°F (20°C to 25°C).
- 2.
- Store mixed CAVERJECT IMPULSE between 36°F to 77°F (2°C to 25°C). Do not freeze.
- 3.
- CAVERJECT IMPULSE should be used within 24 hours after it is mixed.
Keep CAVERJECT IMPULSE and all medicines out of the reach of children.
This Patient Information and Instructions for Use has been approved by the U.S Food and Drug Administration.
LAB-1504-2.0
Revised January 2023
Resources
Didn’t find what you were looking for?
Contact us.
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine.
*Speak with a Pfizer Medical Information Professional regarding your medical inquiry. Available 9AM-5Pm ET Monday to Friday; excluding holidays.
Submit a medical question for Pfizer prescription products.
Report Adverse Event
To report an adverse event related to a Pfizer product and you are not part of a clinical trial* for this product, click the link below to submit your information: Pfizer Safety Reporting Site
*If you are involved in a clinical trial for either product, adverse events should be reported to your coordinating study site.
If you cannot use the above website to report an adverse event related to a Pfizer product, please call Pfizer Medical Information at (800) 438-1985.
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or by calling (800)-332-1088.