butorphanol tartrate injection, USP Clinical Pharmacology
()
CLINICAL PHARMACOLOGY
Mechanism of Action
Butorphanol is a partial opioid agonist at the mu opioid receptor and a full agonist at the kappa opioid receptor. The principal therapeutic action of butorphanol is analgesia. Clinically, dosage is titrated to provide adequate analgesia and may be limited by adverse reactions, including respiratory and CNS depression.
The precise mechanism of the analgesic action is unknown. However, specific CNS opioid receptors for endogenous compounds with opioid-like activity have been identified throughout the brain and spinal cord and are thought to play a role in the analgesic effects of this drug.
Pharmacodynamics
The analgesic effect of butorphanol is influenced by the route of administration. Onset of analgesia is within a few minutes for intravenous administration and within 15 minutes for intramuscular injection.
Peak analgesic activity occurs within 30 to 60 minutes following intravenous and intramuscular administration.
The duration of analgesia varies depending on the pain model as well as the route of administration, but is generally 3 to 4 hours with IM and IV doses as defined by the time 50% of patients required remedication. In postoperative studies, the duration of analgesia with IV or IM butorphanol was similar to morphine, meperidine and pentazocine when administered in the same fashion at equipotent doses [see Clinical Trials].
Effects on the Central Nervous System
Butorphanol produces respiratory depression by direct action on brain stem respiratory centers. The respiratory depression involves a reduction in the responsiveness of the brain stem respiratory centers to both increases in carbon dioxide tension and electrical stimulation.
In human studies involving individuals without significant respiratory dysfunction, 2 mg of butorphanol IV and 10 mg of morphine sulfate IV depressed respiration to a comparable degree. At higher doses, the magnitude of respiratory depression with butorphanol is not appreciably increased; however, the duration of respiratory depression is longer. Respiratory depression noted after administration of butorphanol to humans by any route is reversed by treatment with naloxone, a specific opioid antagonist [see OVERDOSAGE].
Butorphanol, like other mixed agonist-antagonists with a high affinity for the kappa receptor, may produce unpleasant psychotomimetic effects in some individuals.
Nausea and/or vomiting may be produced by doses of 1 mg or more administered by any route.
In human studies of butorphanol [see CLINICAL PHARMACOLOGY; Clinical Trials], sedation is commonly noted at doses of 0.5 mg or more. Narcosis is produced by 10 to 12 mg doses of butorphanol administered over 10 to 15 minutes intravenously.
Butorphanol causes miosis, even in total darkness. Pinpoint pupils are a sign of opioid overdose but are not pathognomonic (e.g., pontine lesions of hemorrhagic or ischemic origins may produce similar findings). Marked mydriasis rather than miosis may be seen due to hypoxia in overdose situations.
Effects on the Gastrointestinal Tract and Other Smooth Muscle
Butorphanol causes a reduction in motility associated with an increase in smooth muscle tone in the antrum of the stomach and duodenum. Digestion of food in the small intestine is delayed and propulsive contractions are decreased. Propulsive peristaltic waves in the colon are decreased, while tone may be increased to the point of spasm, resulting in constipation. Other opioid-induced effects may include a reduction in biliary and pancreatic secretions, spasm of sphincter of Oddi, and transient elevations in serum amylase.
Effects on the Cardiovascular System
Hemodynamic changes noted during cardiac catheterization in patients receiving single 0.025 mg/kg intravenous doses of butorphanol have included increases in pulmonary artery pressure, wedge pressure and vascular resistance, increases in left ventricular end diastolic pressure and in systemic arterial pressure.
Effects on the Endocrine System
Opioids inhibit the secretion of adrenocorticotropic hormone (ACTH), cortisol, and luteinizing hormone (LH) in humans [see ADVERSE REACTIONS]. They also stimulate prolactin, growth hormone (GH) secretion, and pancreatic secretion of insulin and glucagon.
Use of opioids for an extended period of time may influence the hypothalamic-pituitary-gonadal axis, leading to androgen deficiency that may manifest as low libido, impotence, erectile dysfunction, amenorrhea, or infertility. The causal role of opioids in the clinical syndrome of hypogonadism is unknown because the various medical, physical, lifestyle, and psychological stressors that may influence gonadal hormone levels have not been adequately controlled for in studies conducted to date [see ADVERSE REACTIONS].
Effects on the Immune System
Opioids have been shown to have a variety of effects on components of the immune system. The clinical significance of these findings is unknown. Overall, the effects of opioids appear to be modestly immunosuppressive.
Concentration–Efficacy Relationships
The minimum effective analgesic concentration will vary widely among patients, especially among patients who have been previously treated with opioid agonists. The minimum effective analgesic concentration of butorphanol for any individual patient may increase over time due to an increase in pain, the development of a new pain syndrome, and/or the development of analgesic tolerance [see DOSAGE AND ADMINISTRATION].
Concentration–Adverse Reaction Relationships
There is a relationship between increasing butorphanol plasma concentration and increasing frequency of dose-related opioid adverse reactions such as nausea, vomiting, CNS effects, and respiratory depression. In opioid-tolerant patients, the situation may be altered by the development of tolerance to opioid-related adverse reactions [see DOSAGE AND ADMINISTRATION].
Pharmacokinetics
Butorphanol Tartrate Injection is rapidly absorbed after IM injection and peak plasma levels are reached in 20 to 40 minutes.
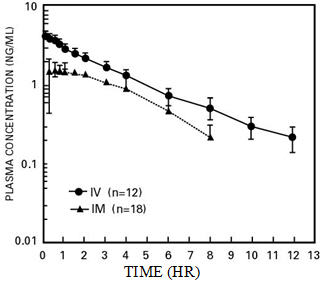
Following its initial absorption/distribution phase, the single-dose pharmacokinetics of butorphanol by the intravenous and intramuscular routes of administration are similar (see Figure 1).
| Figure 1—Butorphanol Plasma Levels After IV and IM Administration of 2 mg Dose |
|---|
 |
Serum protein binding is independent of concentration over the range achieved in clinical practice (up to 7 ng/mL) with a bound fraction of approximately 80%.
The volume of distribution of butorphanol varies from 305 to 901 liters and total body clearance from 52 to 154 liters/hr (see Table 1).
| Parameters | Young | Elderly |
|---|---|---|
AUC (inf)† | 7.24 (1.57)‡ | 8.71 (2.02) |
(hr ∙ ng/mL) | (4.40–9.77)§ | (4.76–13.03) |
Half-life (hr) | 4.56 (1.67) | 5.61 (1.36) |
(2.06–8.70) | (3.25–8.79) | |
Volume of Distribution¶ (L) | 487 (155) | 552 (124) |
Total body | 99 (23) | 82 (21) |
Clearance (L/hr) | (70–154) | (52–143) |
The drug is transported across the blood-brain and placental barriers and into human milk [see PRECAUTIONS: Labor or Delivery and Nursing Mothers].
Butorphanol is extensively metabolized in the liver. Metabolism is qualitatively and quantitatively similar following intravenous or intramuscular administration. Oral bioavailability is only 5 to 17% because of extensive first pass metabolism of butorphanol.
The major metabolite of butorphanol is hydroxybutorphanol, while norbutorphanol is produced in small amounts. Both have been detected in plasma following administration of butorphanol, with norbutorphanol present at trace levels at most time points. The elimination half-life of hydroxybutorphanol is about 18 hours and, as a consequence, considerable accumulation (~5-fold) occurs when butorphanol is dosed to steady state.
Elimination occurs by urine and fecal excretion. When 3H labeled butorphanol is administered to normal subjects, most (70 to 80%) of the dose is recovered in the urine, while approximately 15% is recovered in the feces.
About 5% of the dose is recovered in the urine as butorphanol. Forty-nine percent is eliminated in the urine as hydroxybutorphanol. Less than 5% is excreted in the urine as norbutorphanol.
Butorphanol pharmacokinetics in the elderly differ from younger patients (see Table 1).
In renally impaired patients with creatinine clearances <30 mL/min, the elimination half-life was approximately doubled and the total body clearance was approximately one half (10.5 hours [clearance 150 L/h] compared to 5.8 hours [clearance 260 L/h] in healthy subjects). No effect on Cmax or Tmax was observed after a single-dose.
After intravenous administration to patients with hepatic impairment, the elimination half-life of butorphanol was approximately tripled and total body clearance was approximately one half (half-life 16.8 hours, clearance 92 L/h) compared to healthy subjects (half-life 4.8 hours, clearance 175 L/h). The exposure of hepatically impaired patients to butorphanol was significantly greater (about 2-fold) than that in healthy subjects.
[see PRECAUTIONS: Hepatic and Renal Disease, Drug Interactions and Geriatric Use and CLINICAL PHARMACOLOGY: Individualization of Dosage].
Clinical Trials
The effectiveness of opioid analgesics varies in different pain syndromes. Studies with Butorphanol Tartrate Injection have been performed in postoperative (primarily abdominal and orthopedic) pain and pain during labor and delivery, as preoperative and preanesthetic medication, and as a supplement to balanced anesthesia (see below).
Use in the Management of Pain-Postoperative Pain
The analgesic efficacy of Butorphanol Tartrate Injection in postoperative pain was investigated in several double-blind active-controlled studies involving 958 butorphanol-treated patients. The following doses were found to have approximately equivalent analgesic effect: 2 mg butorphanol, 10 mg morphine, 40 mg pentazocine and 80 mg meperidine.
After intravenous administration of butorphanol tartrate, onset and peak analgesic effect occurred by the time of first observation (30 minutes). After intramuscular administration, pain relief onset occurred at 30 minutes or less, and peak effect occurred between 30 minutes and one hour. The duration of action of Butorphanol Tartrate Injection was 3 to 4 hours when defined as the time necessary for pain intensity to return to pretreatment level or the time to retreatment.
Preanesthetic Medication
Butorphanol Tartrate Injection, (2 mg and 4 mg) and meperidine (80 mg) were studied for use as preanesthetic medication in hospitalized surgical patients. Patients received a single intramuscular dose of either butorphanol or meperidine approximately 90 minutes prior to anesthesia. The anesthesia regimen included barbiturate induction, followed by nitrous oxide and oxygen with halothane or enflurane, with or without a muscle relaxant.
Anesthetic preparation was rated as satisfactory in all 42 butorphanol injection patients regardless of the type of surgery.
Balanced Anesthesia
Butorphanol tartrate administered intravenously (mean dose 2 mg) was compared to intravenous morphine sulfate (mean dose 10 mg) as premedication shortly before thiopental induction, followed by balanced anesthesia in 50 ASA Class 1 and 2 patients. Anesthesia was then maintained by repeated intravenous doses, averaging 4.6 mg butorphanol and 22.8 mg morphine per patient.
Anesthetic induction and maintenance were generally rated as satisfactory with both butorphanol injection (25 patients) and morphine (25 patients) regardless of the type of surgery performed. Emergence from anesthesia was comparable with both agents.
Labor
[see PRECAUTIONS]
The analgesic efficacy of intravenous Butorphanol Tartrate Injection was studied in pain during labor. In a total of 145 patients butorphanol (1 mg and 2 mg) was as effective as 40 mg and 80 mg of meperidine (144 patients) in the relief of pain in labor with no effect on the duration or progress of labor. Both drugs readily crossed the placenta and entered fetal circulation. The condition of the infants in these studies, determined by Apgar scores at 1 and 5 minutes (8 or above) and time to sustained respiration, showed that butorphanol had the same effects on the infants as meperidine.
In these studies neurobehavioral testing in infants exposed to butorphanol injection at a mean of 18.6 hours after delivery, showed no significant differences between treatment groups.
Individualization of Dosage
Use of butorphanol in geriatric patients, patients with renal impairment, patients with hepatic impairment and during labor requires extra caution [see below and the appropriate sections in PRECAUTIONS].
For pain relief the recommended initial dosage regimen of Butorphanol Tartrate Injection is 1 mg IV or 2 mg IM with repeated doses every three to four hours as necessary. This dosage regimen is likely to be effective for the majority of patients. Dosage adjustments of butorphanol injection should be based on observations of its beneficial and adverse effects. The initial dose in the elderly and in patients with renal or hepatic impairment should generally be half the recommended adult dose (0.5 mg IV and 1 mg IM). Repeat doses in these patients should be determined by the patient's response rather than at fixed intervals but will generally be no less than 6 hours [see PRECAUTIONS].
The usual preoperative dose is 2 mg IM given 60 to 90 minutes before surgery or 2 mg IV shortly before induction. This is approximately equivalent in sedative effect to 10 mg morphine or 80 mg of meperidine. This single preoperative dose should be individualized based on age, body weight, physical status, underlying pathological condition, use of other drugs, type of anesthesia to be used and the surgical procedure involved.
During maintenance in balanced anesthesia the usual incremental dose of butorphanol tartrate is 0.5 to 1 mg IV. The incremental dose may be higher, up to 0.06 mg/kg (4 mg/70 kg), depending on previous sedative, analgesic, and hypnotic drugs administered. The total dose of butorphanol injection will vary; however, patients seldom require less than 4 mg or more than 12.5 mg (approximately 0.06 to 0.18 mg/kg).
As with other opioids of this class, butorphanol injection may not provide adequate intraoperative analgesia in every patient or under all conditions. A failure to achieve successful analgesia during balanced anesthesia is commonly reflected by increases in general sympathetic tone. Consequently, if blood pressure or heart rate continue to rise, consideration should be given to adding a potent volatile liquid inhalation anesthetic or another intravenous medication.
In labor, the recommended initial dose of butorphanol tartrate is 1 or 2 mg IM or IV in mothers with fetuses of 37 weeks gestation or beyond and without signs of fetal distress. Dosage adjustments of butorphanol in labor should be based on initial response with consideration given to concomitant analgesic or sedative drugs and the expected time of delivery. A dose should not be repeated in less than four hours nor administered less than four hours prior to the anticipated delivery [see PRECAUTIONS].
Find butorphanol tartrate injection, USP medical information:
Find butorphanol tartrate injection, USP medical information:
butorphanol tartrate injection, USP Quick Finder
Health Professional Information
Clinical Pharmacology
CLINICAL PHARMACOLOGY
Mechanism of Action
Butorphanol is a partial opioid agonist at the mu opioid receptor and a full agonist at the kappa opioid receptor. The principal therapeutic action of butorphanol is analgesia. Clinically, dosage is titrated to provide adequate analgesia and may be limited by adverse reactions, including respiratory and CNS depression.
The precise mechanism of the analgesic action is unknown. However, specific CNS opioid receptors for endogenous compounds with opioid-like activity have been identified throughout the brain and spinal cord and are thought to play a role in the analgesic effects of this drug.
Pharmacodynamics
The analgesic effect of butorphanol is influenced by the route of administration. Onset of analgesia is within a few minutes for intravenous administration and within 15 minutes for intramuscular injection.
Peak analgesic activity occurs within 30 to 60 minutes following intravenous and intramuscular administration.
The duration of analgesia varies depending on the pain model as well as the route of administration, but is generally 3 to 4 hours with IM and IV doses as defined by the time 50% of patients required remedication. In postoperative studies, the duration of analgesia with IV or IM butorphanol was similar to morphine, meperidine and pentazocine when administered in the same fashion at equipotent doses [see Clinical Trials].
Effects on the Central Nervous System
Butorphanol produces respiratory depression by direct action on brain stem respiratory centers. The respiratory depression involves a reduction in the responsiveness of the brain stem respiratory centers to both increases in carbon dioxide tension and electrical stimulation.
In human studies involving individuals without significant respiratory dysfunction, 2 mg of butorphanol IV and 10 mg of morphine sulfate IV depressed respiration to a comparable degree. At higher doses, the magnitude of respiratory depression with butorphanol is not appreciably increased; however, the duration of respiratory depression is longer. Respiratory depression noted after administration of butorphanol to humans by any route is reversed by treatment with naloxone, a specific opioid antagonist [see OVERDOSAGE].
Butorphanol, like other mixed agonist-antagonists with a high affinity for the kappa receptor, may produce unpleasant psychotomimetic effects in some individuals.
Nausea and/or vomiting may be produced by doses of 1 mg or more administered by any route.
In human studies of butorphanol [see CLINICAL PHARMACOLOGY; Clinical Trials], sedation is commonly noted at doses of 0.5 mg or more. Narcosis is produced by 10 to 12 mg doses of butorphanol administered over 10 to 15 minutes intravenously.
Butorphanol causes miosis, even in total darkness. Pinpoint pupils are a sign of opioid overdose but are not pathognomonic (e.g., pontine lesions of hemorrhagic or ischemic origins may produce similar findings). Marked mydriasis rather than miosis may be seen due to hypoxia in overdose situations.
Effects on the Gastrointestinal Tract and Other Smooth Muscle
Butorphanol causes a reduction in motility associated with an increase in smooth muscle tone in the antrum of the stomach and duodenum. Digestion of food in the small intestine is delayed and propulsive contractions are decreased. Propulsive peristaltic waves in the colon are decreased, while tone may be increased to the point of spasm, resulting in constipation. Other opioid-induced effects may include a reduction in biliary and pancreatic secretions, spasm of sphincter of Oddi, and transient elevations in serum amylase.
Effects on the Cardiovascular System
Hemodynamic changes noted during cardiac catheterization in patients receiving single 0.025 mg/kg intravenous doses of butorphanol have included increases in pulmonary artery pressure, wedge pressure and vascular resistance, increases in left ventricular end diastolic pressure and in systemic arterial pressure.
Effects on the Endocrine System
Opioids inhibit the secretion of adrenocorticotropic hormone (ACTH), cortisol, and luteinizing hormone (LH) in humans [see ADVERSE REACTIONS]. They also stimulate prolactin, growth hormone (GH) secretion, and pancreatic secretion of insulin and glucagon.
Use of opioids for an extended period of time may influence the hypothalamic-pituitary-gonadal axis, leading to androgen deficiency that may manifest as low libido, impotence, erectile dysfunction, amenorrhea, or infertility. The causal role of opioids in the clinical syndrome of hypogonadism is unknown because the various medical, physical, lifestyle, and psychological stressors that may influence gonadal hormone levels have not been adequately controlled for in studies conducted to date [see ADVERSE REACTIONS].
Effects on the Immune System
Opioids have been shown to have a variety of effects on components of the immune system. The clinical significance of these findings is unknown. Overall, the effects of opioids appear to be modestly immunosuppressive.
Concentration–Efficacy Relationships
The minimum effective analgesic concentration will vary widely among patients, especially among patients who have been previously treated with opioid agonists. The minimum effective analgesic concentration of butorphanol for any individual patient may increase over time due to an increase in pain, the development of a new pain syndrome, and/or the development of analgesic tolerance [see DOSAGE AND ADMINISTRATION].
Concentration–Adverse Reaction Relationships
There is a relationship between increasing butorphanol plasma concentration and increasing frequency of dose-related opioid adverse reactions such as nausea, vomiting, CNS effects, and respiratory depression. In opioid-tolerant patients, the situation may be altered by the development of tolerance to opioid-related adverse reactions [see DOSAGE AND ADMINISTRATION].
Pharmacokinetics
Butorphanol Tartrate Injection is rapidly absorbed after IM injection and peak plasma levels are reached in 20 to 40 minutes.
Following its initial absorption/distribution phase, the single-dose pharmacokinetics of butorphanol by the intravenous and intramuscular routes of administration are similar (see Figure 1).
| Figure 1—Butorphanol Plasma Levels After IV and IM Administration of 2 mg Dose |
|---|
 |
Serum protein binding is independent of concentration over the range achieved in clinical practice (up to 7 ng/mL) with a bound fraction of approximately 80%.
The volume of distribution of butorphanol varies from 305 to 901 liters and total body clearance from 52 to 154 liters/hr (see Table 1).
| Parameters | Young | Elderly |
|---|---|---|
AUC (inf)† | 7.24 (1.57)‡ | 8.71 (2.02) |
(hr ∙ ng/mL) | (4.40–9.77)§ | (4.76–13.03) |
Half-life (hr) | 4.56 (1.67) | 5.61 (1.36) |
(2.06–8.70) | (3.25–8.79) | |
Volume of Distribution¶ (L) | 487 (155) | 552 (124) |
Total body | 99 (23) | 82 (21) |
Clearance (L/hr) | (70–154) | (52–143) |
The drug is transported across the blood-brain and placental barriers and into human milk [see PRECAUTIONS: Labor or Delivery and Nursing Mothers].
Butorphanol is extensively metabolized in the liver. Metabolism is qualitatively and quantitatively similar following intravenous or intramuscular administration. Oral bioavailability is only 5 to 17% because of extensive first pass metabolism of butorphanol.
The major metabolite of butorphanol is hydroxybutorphanol, while norbutorphanol is produced in small amounts. Both have been detected in plasma following administration of butorphanol, with norbutorphanol present at trace levels at most time points. The elimination half-life of hydroxybutorphanol is about 18 hours and, as a consequence, considerable accumulation (~5-fold) occurs when butorphanol is dosed to steady state.
Elimination occurs by urine and fecal excretion. When 3H labeled butorphanol is administered to normal subjects, most (70 to 80%) of the dose is recovered in the urine, while approximately 15% is recovered in the feces.
About 5% of the dose is recovered in the urine as butorphanol. Forty-nine percent is eliminated in the urine as hydroxybutorphanol. Less than 5% is excreted in the urine as norbutorphanol.
Butorphanol pharmacokinetics in the elderly differ from younger patients (see Table 1).
In renally impaired patients with creatinine clearances <30 mL/min, the elimination half-life was approximately doubled and the total body clearance was approximately one half (10.5 hours [clearance 150 L/h] compared to 5.8 hours [clearance 260 L/h] in healthy subjects). No effect on Cmax or Tmax was observed after a single-dose.
After intravenous administration to patients with hepatic impairment, the elimination half-life of butorphanol was approximately tripled and total body clearance was approximately one half (half-life 16.8 hours, clearance 92 L/h) compared to healthy subjects (half-life 4.8 hours, clearance 175 L/h). The exposure of hepatically impaired patients to butorphanol was significantly greater (about 2-fold) than that in healthy subjects.
[see PRECAUTIONS: Hepatic and Renal Disease, Drug Interactions and Geriatric Use and CLINICAL PHARMACOLOGY: Individualization of Dosage].
Clinical Trials
The effectiveness of opioid analgesics varies in different pain syndromes. Studies with Butorphanol Tartrate Injection have been performed in postoperative (primarily abdominal and orthopedic) pain and pain during labor and delivery, as preoperative and preanesthetic medication, and as a supplement to balanced anesthesia (see below).
Use in the Management of Pain-Postoperative Pain
The analgesic efficacy of Butorphanol Tartrate Injection in postoperative pain was investigated in several double-blind active-controlled studies involving 958 butorphanol-treated patients. The following doses were found to have approximately equivalent analgesic effect: 2 mg butorphanol, 10 mg morphine, 40 mg pentazocine and 80 mg meperidine.
After intravenous administration of butorphanol tartrate, onset and peak analgesic effect occurred by the time of first observation (30 minutes). After intramuscular administration, pain relief onset occurred at 30 minutes or less, and peak effect occurred between 30 minutes and one hour. The duration of action of Butorphanol Tartrate Injection was 3 to 4 hours when defined as the time necessary for pain intensity to return to pretreatment level or the time to retreatment.
Preanesthetic Medication
Butorphanol Tartrate Injection, (2 mg and 4 mg) and meperidine (80 mg) were studied for use as preanesthetic medication in hospitalized surgical patients. Patients received a single intramuscular dose of either butorphanol or meperidine approximately 90 minutes prior to anesthesia. The anesthesia regimen included barbiturate induction, followed by nitrous oxide and oxygen with halothane or enflurane, with or without a muscle relaxant.
Anesthetic preparation was rated as satisfactory in all 42 butorphanol injection patients regardless of the type of surgery.
Balanced Anesthesia
Butorphanol tartrate administered intravenously (mean dose 2 mg) was compared to intravenous morphine sulfate (mean dose 10 mg) as premedication shortly before thiopental induction, followed by balanced anesthesia in 50 ASA Class 1 and 2 patients. Anesthesia was then maintained by repeated intravenous doses, averaging 4.6 mg butorphanol and 22.8 mg morphine per patient.
Anesthetic induction and maintenance were generally rated as satisfactory with both butorphanol injection (25 patients) and morphine (25 patients) regardless of the type of surgery performed. Emergence from anesthesia was comparable with both agents.
Labor
[see PRECAUTIONS]
The analgesic efficacy of intravenous Butorphanol Tartrate Injection was studied in pain during labor. In a total of 145 patients butorphanol (1 mg and 2 mg) was as effective as 40 mg and 80 mg of meperidine (144 patients) in the relief of pain in labor with no effect on the duration or progress of labor. Both drugs readily crossed the placenta and entered fetal circulation. The condition of the infants in these studies, determined by Apgar scores at 1 and 5 minutes (8 or above) and time to sustained respiration, showed that butorphanol had the same effects on the infants as meperidine.
In these studies neurobehavioral testing in infants exposed to butorphanol injection at a mean of 18.6 hours after delivery, showed no significant differences between treatment groups.
Individualization of Dosage
Use of butorphanol in geriatric patients, patients with renal impairment, patients with hepatic impairment and during labor requires extra caution [see below and the appropriate sections in PRECAUTIONS].
For pain relief the recommended initial dosage regimen of Butorphanol Tartrate Injection is 1 mg IV or 2 mg IM with repeated doses every three to four hours as necessary. This dosage regimen is likely to be effective for the majority of patients. Dosage adjustments of butorphanol injection should be based on observations of its beneficial and adverse effects. The initial dose in the elderly and in patients with renal or hepatic impairment should generally be half the recommended adult dose (0.5 mg IV and 1 mg IM). Repeat doses in these patients should be determined by the patient's response rather than at fixed intervals but will generally be no less than 6 hours [see PRECAUTIONS].
The usual preoperative dose is 2 mg IM given 60 to 90 minutes before surgery or 2 mg IV shortly before induction. This is approximately equivalent in sedative effect to 10 mg morphine or 80 mg of meperidine. This single preoperative dose should be individualized based on age, body weight, physical status, underlying pathological condition, use of other drugs, type of anesthesia to be used and the surgical procedure involved.
During maintenance in balanced anesthesia the usual incremental dose of butorphanol tartrate is 0.5 to 1 mg IV. The incremental dose may be higher, up to 0.06 mg/kg (4 mg/70 kg), depending on previous sedative, analgesic, and hypnotic drugs administered. The total dose of butorphanol injection will vary; however, patients seldom require less than 4 mg or more than 12.5 mg (approximately 0.06 to 0.18 mg/kg).
As with other opioids of this class, butorphanol injection may not provide adequate intraoperative analgesia in every patient or under all conditions. A failure to achieve successful analgesia during balanced anesthesia is commonly reflected by increases in general sympathetic tone. Consequently, if blood pressure or heart rate continue to rise, consideration should be given to adding a potent volatile liquid inhalation anesthetic or another intravenous medication.
In labor, the recommended initial dose of butorphanol tartrate is 1 or 2 mg IM or IV in mothers with fetuses of 37 weeks gestation or beyond and without signs of fetal distress. Dosage adjustments of butorphanol in labor should be based on initial response with consideration given to concomitant analgesic or sedative drugs and the expected time of delivery. A dose should not be repeated in less than four hours nor administered less than four hours prior to the anticipated delivery [see PRECAUTIONS].
Resources
Didn’t find what you were looking for? Contact us.
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine.
*Speak with a Pfizer Medical Information Professional regarding your medical inquiry. Available 9AM-5Pm ET Monday to Friday; excluding holidays.
Submit a medical question for Pfizer prescription products.
Report Adverse Event
To report an adverse event related to the Pfizer-BioNTech COVID-19 Vaccine, and you are not part of a clinical trial* for this product, click the link below to submit your information:
Pfizer Safety Reporting Site*If you are involved in a clinical trial for this product, adverse events should be reported to your coordinating study site.
If you cannot use the above website, or would like to report an adverse event related to a different Pfizer product, please call Pfizer Safety at (800) 438-1985.
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or call (800) 822-7967.