bortezomib injection 3.5 MG VIAL Clinical Studies
(bortezomib for injection)
14 CLINICAL STUDIES
14.1 Multiple Myeloma
Randomized, Open-Label Clinical Study in Patients with Previously Untreated Multiple Myeloma
A prospective, international, randomized (1:1), open-label clinical study (NCT00111319) of 682 patients was conducted to determine whether bortezomib for injection administered intravenously (1.3 mg/m2) in combination with melphalan (9 mg/m2) and prednisone (60 mg/m2) resulted in improvement in time to progression (TTP) when compared to melphalan (9 mg/m2) and prednisone (60 mg/m2) in patients with previously untreated multiple myeloma. Treatment was administered for a maximum of nine cycles (approximately 54 weeks) and was discontinued early for disease progression or unacceptable toxicity. Antiviral prophylaxis was recommended for patients on the bortezomib for injection study arm.
The median age of the patients in the study was 71 years (48;91), 50% were male, 88% were Caucasian and the median Karnofsky performance status score for the patients was 80 (60;100). Patients had IgG/IgA/Light chain myeloma in 63%/25%/8% instances, a median hemoglobin of 105 g/L (64;165), and a median platelet count of 221,500/microliter (33,000;587,000).
Efficacy results for the trial are presented in Table 14. At a prespecified interim analysis (with median follow-up of 16.3 months), the combination of bortezomib for injection, melphalan and prednisone therapy resulted in significantly superior results for time to progression, progression-free survival, overall survival and response rate. Further enrollment was halted, and patients receiving melphalan and prednisone were offered bortezomib for injection in addition. A later, prespecified analysis of overall survival (with median follow-up of 36.7 months with a hazard ratio of 0.65, 95% CI: 0.51, 0.84) resulted in a statistically significant survival benefit for the bortezomib for injection, melphalan and prednisone treatment arm despite subsequent therapies including bortezomib for injection based regimens. In an updated analysis of overall survival based on 387 deaths (median follow-up 60.1 months), the median overall survival for the bortezomib for injection, melphalan and prednisone treatment arm was 56.4 months and for the melphalan and prednisone treatment arm was 43.1 months, with a hazard ratio of 0.695 (95% CI: 0.57, 0.85).
| Efficacy Endpoint | Bortezomib for Injection, Melphalan and Prednisone (n=344) | Melphalan and Prednisone (n=338) |
|---|---|---|
| Note: All results are based on the analysis performed at a median follow-up duration of 16.3 months except for the overall survival analysis. | ||
| ||
| Time to Progression | ||
| Events n (%) | 101 (29) | 152 (45) |
| Median* (months) | 20.7 | 15.0 |
| (95% CI) | (17.6, 24.7) | (14.1, 17.9) |
| Hazard ratio † | 0.54 | |
| (95% CI) | (0.42, 0.70) | |
| p-value ‡ | 0.000002 | |
| Progression-Free Survival | ||
| Events n (%) | 135 (39) | 190 (56) |
| Median* (months) | 18.3 | 14.0 |
| (95% CI) | (16.6, 21.7) | (11.1, 15.0) |
| Hazard ratio† | 0.61 | |
| (95% CI) | (0.49, 0.76) | |
| p-value‡ | 0.00001 | |
| Response Rate | ||
| CR§ n (%) | 102 (30) | 12 (4) |
| PR§ n (%) | 136 (40) | 103 (30) |
| nCR n (%) | 5 (1) | 0 |
| CR + PR§ n (%) | 238 (69) | 115 (34) |
| p-value¶ | <10-10 | |
| Overall Survival at Median Follow-Up of 36.7 Months | ||
| Events (deaths) n (%) | 109 (32) | 148 (44) |
| Median* (months) | Not Reached | 43.1 |
| (95% CI) | (46.2, NR) | (34.8, NR) |
| Hazard ratio† | 0.65 | |
| (95% CI) | (0.51, 0.84) | |
| p-value‡ | 0.00084 | |
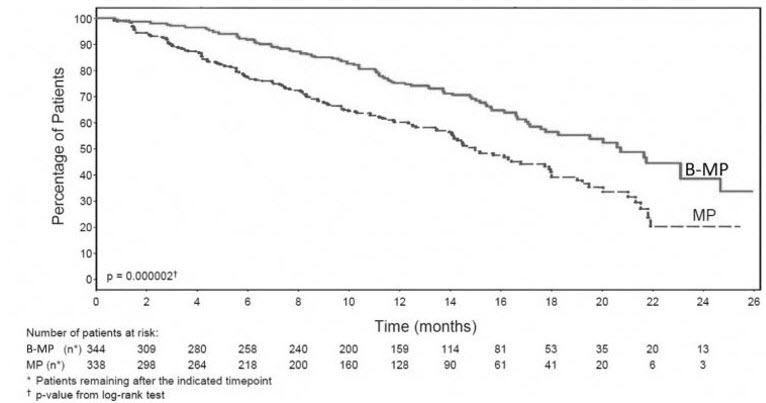
TTP was statistically significantly longer on the bortezomib for injection, melphalan and prednisone arm (see Figure 1). (median follow-up 16.3 months)
 |
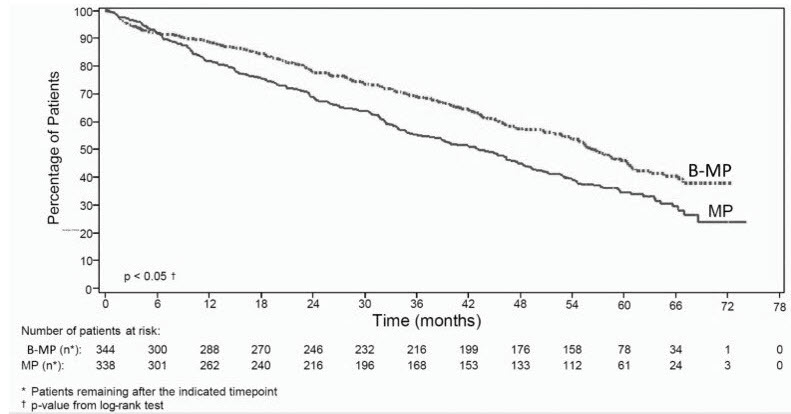
Overall survival was statistically significantly longer on the bortezomib for injection, melphalan and prednisone arm (see Figure 2). (median follow-up 60.1 months)
 |
Randomized, Clinical Study in Relapsed Multiple Myeloma of Bortezomib for Injection vs Dexamethasone
A prospective Phase 3, international, randomized (1:1), stratified, open-label clinical study (NCT00048230) enrolling 669 patients was designed to determine whether bortezomib for injection resulted in improvement in time to progression (TTP) compared to high-dose dexamethasone in patients with progressive multiple myeloma following 1 to 3 prior therapies. Patients considered to be refractory to prior high-dose dexamethasone were excluded as were those with baseline Grade ≥2 peripheral neuropathy or platelet counts <50,000/μL. A total of 627 patients were evaluable for response.
Stratification factors were based on the number of lines of prior therapy the patient had previously received (one previous line vs more than one line of therapy), time of progression relative to prior treatment (progression during or within six months of stopping their most recent therapy vs relapse >6 months after receiving their most recent therapy), and screening beta2-microglobulin levels (≤2.5 mg/L vs >2.5 mg/L). Baseline patient and disease characteristics are summarized in Table 15.
| Patient Characteristics | Bortezomib for Injection (N=333) | Dexamethasone (N=336) |
|---|---|---|
| Median age in years (range) | 62.0 (33, 84) | 61.0 (27, 86) |
| Gender: Male/female | 56% / 44% | 60% / 40% |
| Race: Caucasian/black/other | 90% / 6% / 4% | 88% / 7% / 5% |
| Karnofsky performance status score ≤70 | 13% | 17% |
| Hemoglobin <100 g/L | 32% | 28% |
| Platelet count <75 × 109/L | 6% | 4% |
| Disease Characteristics | ||
| Type of myeloma (%): IgG/IgA/Light chain | 60% / 23% / 12% | 59% / 24% / 13% |
| Median beta2-microglobulin (mg/L) | 3.7 | 3.6 |
| Median albumin (g/L) | 39.0 | 39.0 |
| Creatinine clearance ≤30 mL/min [n (%)] | 17 (5%) | 11 (3%) |
| Median Duration of Multiple Myeloma Since Diagnosis (Years) | 3.5 | 3.1 |
| Number of Prior Therapeutic Lines of Treatment | ||
| Median | 2 | 2 |
| 1 prior line | 40% | 35% |
| >1 prior line | 60% | 65% |
| Previous Therapy | ||
| Any prior steroids, e.g., dexamethasone, VAD | 98% | 99% |
| Any prior anthracyclines, e.g., VAD, mitoxantrone | 77% | 76% |
| Any prior alkylating agents, e.g., MP, VBMCP | 91% | 92% |
| Any prior thalidomide therapy | 48% | 50% |
| Vinca alkaloids | 74% | 72% |
| Prior stem cell transplant/other high-dose therapy | 67% | 68% |
| Prior experimental or other types of therapy | 3% | 2% |
Patients in the bortezomib for injection treatment group were to receive 8, three week treatment cycles followed by 3, five week treatment cycles of bortezomib for injection. Patients achieving a CR were treated for four cycles beyond first evidence of CR. Within each three week treatment cycle, bortezomib for injection 1.3 mg/m2/dose alone was administered by intravenous bolus twice weekly for two weeks on Days 1, 4, 8, and 11 followed by a ten day rest period (Days 12 to 21). Within each five week treatment cycle, bortezomib for injection 1.3 mg/m2/dose alone was administered by intravenous bolus once weekly for four weeks on Days 1, 8, 15, and 22 followed by a 13 day rest period (Days 23 to 35) [see Dosage and Administration (2.2)].
Patients in the dexamethasone treatment group were to receive 4, five week treatment cycles followed by 5, four week treatment cycles. Within each five week treatment cycle, dexamethasone 40 mg/day PO was administered once daily on Days 1 to 4, 9 to 12, and 17 to 20 followed by a 15 day rest period (Days 21 to 35). Within each four week treatment cycle, dexamethasone 40 mg/day PO was administered once daily on Days 1 to 4 followed by a 24 day rest period (Days 5 to 28). Patients with documented progressive disease on dexamethasone were offered bortezomib for injection at a standard dose and schedule on a companion study. Following a preplanned interim analysis of time to progression, the dexamethasone arm was halted and all patients randomized to dexamethasone were offered bortezomib for injection, regardless of disease status.
In the bortezomib for injection arm, 34% of patients received at least one bortezomib for injection dose in all eight of the three week cycles of therapy, and 13% received at least one dose in all 11 cycles. The average number of bortezomib for injection doses during the study was 22, with a range of 1 to 44. In the dexamethasone arm, 40% of patients received at least one dose in all four of the five week treatment cycles of therapy, and 6% received at least one dose in all nine cycles.
The time to event analyses and response rates from the relapsed multiple myeloma study are presented in Table 16. Response and progression were assessed using the European Group for Blood and Marrow Transplantation (EBMT) criteria. Complete response (CR) required <5% plasma cells in the marrow, 100% reduction in M-protein, and a negative immunofixation test (IF-). Partial response (PR) requires ≥50% reduction in serum myeloma protein and ≥90% reduction of urine myeloma protein on at least two occasions for a minimum of at least six weeks along with stable bone disease and normal calcium. Near complete response (nCR) was defined as meeting all the criteria for complete response including 100% reduction in M-protein by protein electrophoresis; however, M-protein was still detectable by immunofixation (IF+).
| Efficacy Endpoint | All Patients | 1 Prior Line of Therapy | >1 Prior Line of Therapy | |||
|---|---|---|---|---|---|---|
| Bortezomib | Dex | Bortezomib | Dex | Bortezomib | Dex | |
| (n=333) | (n=336) | (n=132) | (n=119) | (n=200) | (n=217) | |
| ||||||
| Time to Progression Events n (%) | 147 (44) | 196 (58) | 55 (42) | 64 (54) | 92 (46) | 132 (61) |
| Median* (95% CI) | 6.2 mo (4.9, 6.9) | 3.5 mo (2.9, 4.2) | 7.0 mo (6.2, 8.8) | 5.6 mo (3.4, 6.3) | 4.9 mo (4.2, 6.3) | 2.9 mo (2.8, 3.5) |
| Hazard ratio† (95% CI) | 0.55 (0.44, 0.69) | 0.55 (0.38, 0.81) | 0.54 (0.41, 0.72) | |||
| p-value‡ | <0.0001 | 0.0019 | <0.0001 | |||
| Overall Survival Events (deaths) n (%) | 51 (15) | 84 (25) | 12 (9) | 24 (20) | 39 (20) | 60 (28) |
| Hazard ratio† (95% CI) | 0.57 (0.40, 0.81) | 0.39 (0.19, 0.81) | 0.65 (0.43, 0.97) | |||
| p-value‡, § | <0.05 | <0.05 | <0.05 | |||
| Response Rate Population¶ n=627 | n=315 | n=312 | n=128 | n=110 | n=187 | n=202 |
| CR# n (%) | 20 (6) | 2 (<1) | 8 (6) | 2 (2) | 12 (6) | 0 (0) |
| PR# n (%) | 101 (32) | 54 (17) | 49 (38) | 27 (25) | 52 (28) | 27 (13) |
| nCR#,Þ n (%) | 21 (7) | 3 (<1) | 8 (6) | 2 (2) | 13 (7) | 1 (<1) |
| CR + PR# n (%) | 121 (38) | 56 (18) | 57 (45) | 29 (26) | 64 (34) | 27 (13) |
| p-valueß | <0.0001 | 0.0035 | <0.0001 | |||
TTP was statistically significantly longer on the bortezomib for injection arm (see Figure 3).
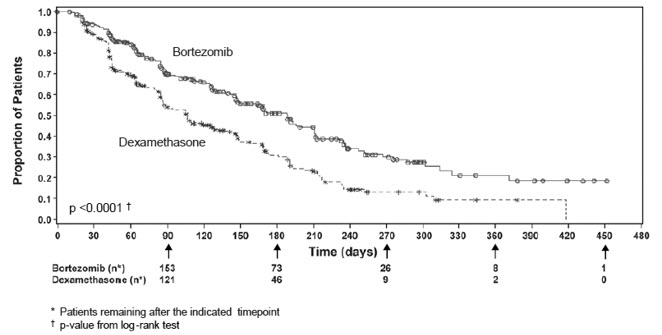 |
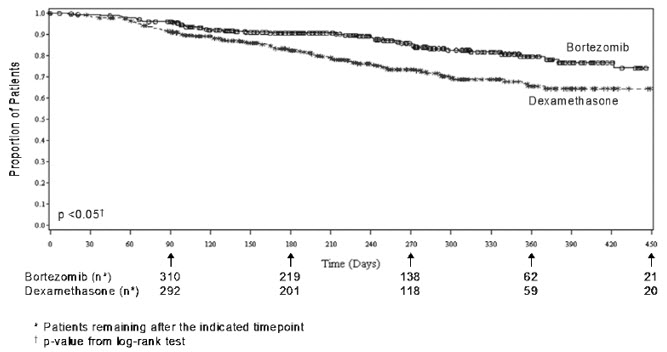
As shown in Figure 4, bortezomib for injection had a significant survival advantage relative to dexamethasone (p <0.05). The median follow-up was 8.3 months.
 |
For the 121 patients achieving a response (CR or PR) on the bortezomib for injection arm, the median duration was 8.0 months (95% CI: 6.9, 11.5 months) compared to 5.6 months (95% CI: 4.8, 9.2 months) for the 56 responders on the dexamethasone arm. The response rate was significantly higher on the bortezomib for injection arm regardless of beta2-microglobulin levels at baseline.
Randomized, Open-Label Clinical Study of Bortezomib for Injection Subcutaneous vs Intravenous in Relapsed Multiple Myeloma
An open-label, randomized, Phase 3 noninferiority study (NCT00722566) compared the efficacy and safety of the subcutaneous administration of bortezomib for injection vs the intravenous administration. This study included 222 bortezomib naïve patients with relapsed multiple myeloma, who were randomized in a 2:1 ratio to receive 1.3 mg/m2 of bortezomib for injection by either the subcutaneous (n=148) or intravenous (n=74) route for eight cycles. Patients who did not obtain an optimal response (less than Complete Response (CR)) to therapy with bortezomib for injection alone after four cycles were allowed to receive oral dexamethasone 20 mg daily on the day of and after bortezomib for injection administration (82 patients in subcutaneous treatment group and 39 patients in the intravenous treatment group). Patients with baseline Grade ≥2 peripheral neuropathy or neuropathic pain, or platelet counts <50,000/μL were excluded. A total of 218 patients were evaluable for response.
Stratification factors were based on the number of lines of prior therapy the patient had received (one previous line vs more than one line of therapy), and international staging system (ISS) stage (incorporating beta2-microglobulin and albumin levels; Stages I, II, or III).
The baseline demographic and other characteristics of the two treatment groups are summarized as follows: the median age of the patient population was approximately 64 years of age (range: 38 to 88 years), primarily male (subcutaneous: 50%, intravenous: 64%); the primary type of myeloma is IgG (subcutaneous: 65% IgG, 26% IgA, 8% light chain; intravenous: 72% IgG, 19% IgA, 8% light chain), ISS staging I/II/III (%) was 27, 41, 32 for both subcutaneous and intravenous, Karnofsky performance status score was ≤70% in 22% of subcutaneous and 16% of intravenous, creatinine clearance was 67.5 mL/min in subcutaneous and 73 mL/min in intravenous, the median years from diagnosis was 2.68 and 2.93 in subcutaneous and intravenous respectively and the proportion of patients with more than one prior line of therapy was 38% in subcutaneous and 35% in intravenous.
This study met its primary (noninferiority) objective that single agent subcutaneous bortezomib for injection retains at least 60% of the overall response rate after four cycles relative to single agent intravenous bortezomib for injection. The results are provided in Table 17.
| Subcutaneous Bortezomib | Intravenous Bortezomib | |
|---|---|---|
| Intent to Treat Population | (n=148) | (n=74) |
| ||
| Primary Endpoint | ||
| Response Rate at 4 Cycles | ||
| ORR (CR + PR) n(%) | 63 (43) | 31 (42) |
| Ratio of Response Rates (95% CI) | 1.01 (0.73, 1.40) | |
| CR n (%) | 11 (7) | 6 (8) |
| PR n (%) | 52 (35) | 25 (34) |
| nCR n (%) | 9 (6) | 4 (5) |
| Secondary Endpoints | ||
| Response Rate at 8 Cycles | ||
| ORR (CR + PR) | 78 (53) | 38 (51) |
| CR n (%) | 17 (11) | 9 (12) |
| PR n (%) | 61 (41) | 29 (39) |
| nCR n (%) | 14 (9) | 7 (9) |
| Median Time to Progression, months | 10.4 | 9.4 |
| Median Progression-Free Survival, months | 10.2 | 8.0 |
| 1 Year Overall Survival (%)* | 72.6 | 76.7 |
A Randomized, Phase 2 Dose-Response Study in Relapsed Multiple Myeloma
An open-label, multicenter study randomized 54 patients with multiple myeloma who had progressed or relapsed on or after front-line therapy to receive bortezomib for injection 1 mg/m2 or 1.3 mg/m2 intravenous bolus twice weekly for two weeks on Days 1, 4, 8, and 11 followed by a ten day rest period (Days 12 to 21). The median duration of time between diagnosis of multiple myeloma and first dose of bortezomib for injection on this trial was two years, and patients had received a median of one prior line of treatment (median of three prior therapies). A single complete response was seen at each dose. The overall response rates (CR + PR) were 30% (8/27) at 1 mg/m2 and 38% (10/26) at 1.3 mg/m2 .
A Phase 2 Open-Label Extension Study in Relapsed Multiple Myeloma
Patients from the two Phase 2 studies, who in the investigators' opinion would experience additional clinical benefit, continued to receive bortezomib for injection beyond 8 cycles on an extension study. Sixty-three (63) patients from the Phase 2 multiple myeloma studies were enrolled and received a median of seven additional cycles of bortezomib for injection therapy for a total median of 14 cycles (range: 7 to 32). The overall median dosing intensity was the same in both the parent protocol and extension study. Sixty-seven percent (67%) of patients initiated the extension study at the same or higher dose intensity at which they completed the parent protocol, and 89% of patients maintained the standard three week dosing schedule during the extension study. No new cumulative or new long-term toxicities were observed with prolonged bortezomib for injection treatment [see Adverse Reactions (6.1)].
A Single-Arm Trial of Retreatment in Relapsed Multiple Myeloma
A single-arm, open-label trial (NCT00431769) was conducted to determine the efficacy and safety of retreatment with bortezomib for injection. One hundred and thirty patients (≥18 years of age) with multiple myeloma who previously had at least partial response on a bortezomib for injection-containing regimen (median of two prior lines of therapy [range: 1–7]) were retreated upon progression with bortezomib for injection administered intravenously. Patients were excluded from trial participation if they had peripheral neuropathy or neuropathic pain of Grade ≥2. At least six months after prior bortezomib for injection therapy, bortezomib for injection was restarted at the last tolerated dose of 1.3 mg/m2 (n=93) or ≤1.0 mg/m2 (n=37) and given on Days 1, 4, 8 and 11 every three weeks for maximum of eight cycles either as single agent or in combination with dexamethasone in accordance with the standard of care. Dexamethasone was administered in combination with bortezomib for injection to 83 patients in Cycle 1 with an additional 11 patients receiving dexamethasone during the course of bortezomib for injection retreatment cycles.
The primary endpoint was best confirmed response to retreatment as assessed by European Group for Blood and Marrow Transplantation (EBMT) criteria. Fifty of the 130 patients achieved a best confirmed response of Partial Response or better for an overall response rate of 38.5% (95% CI: 30.1, 47.4). One patient achieved a Complete Response and 49 achieved Partial Response. In the 50 responding patients, the median duration of response was 6.5 months and the range was 0.6 to 19.3 months.
14.2 Mantle Cell Lymphoma
A Randomized, Open-Label Clinical Study in Patients with Previously Untreated Mantle Cell Lymphoma
A randomized, open-label, Phase 3 study (NCT00722137) was conducted in 487 adult patients with previously untreated mantle cell lymphoma (Stage II, III or IV) who were ineligible or not considered for bone marrow transplantation to determine whether bortezomib administered in combination with rituximab, cyclophosphamide, doxorubicin, and prednisone (VcR-CAP) resulted in improvement in progression-free survival (PFS) when compared to the combination of rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP). This clinical study utilized independent pathology confirmation and independent radiologic response assessment.
Patients in the VcR-CAP treatment arm received bortezomib (1.3 mg/m2) administered intravenously on Days 1, 4, 8, and 11 (rest period Days 12 to 21); rituximab (375 mg/m2) on Day 1; cyclophosphamide (750 mg/m2) on Day 1; doxorubicin (50 mg/m2) on Day 1; and prednisone (100 mg/m2) on Day 1 through Day 5 of the 21 day treatment cycle. For patients with a response first documented at Cycle 6, two additional treatment cycles were allowed.
Median patient age was 66 years, 74% were male, 66% were Caucasian and 32% were Asian. Sixty-nine percent of patients had a positive bone marrow aspirate and/or a positive bone marrow biopsy for MCL, 54% of patients had an International Prognostic Index (IPI) score of three (high-intermediate) or higher and 76% had Stage IV disease.
The majority of the patients in both groups received six or more cycles of treatment, 84% in the VcR-CAP group and 83% in the R-CHOP group. Median number of cycles received by patients in both treatment arms was six with 17% of patients in the R-CHOP group and 14% of subjects in the VcR-CAP group receiving up to two additional cycles.
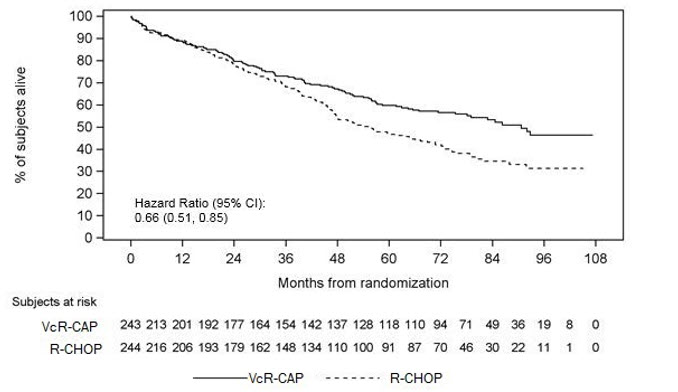
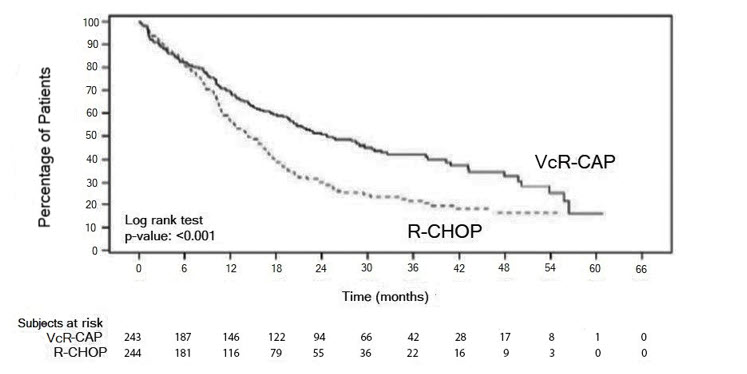
The efficacy results for PFS, CR and ORR with a median follow-up of 40 months are presented in Table 18. The response criteria used to assess efficacy were based on the International Workshop to Standardize Response Criteria for Non-Hodgkin's Lymphoma (IWRC). Final overall survival results at a median follow-up of 78.5 months are also presented in Table 18 and Figure 6. The combination of VcR-CAP resulted in statistically significant prolongation of PFS compared with R-CHOP (see Table 18, Figure 5).
| Efficacy Endpoint n: Intent to Treat patients | VcR-CAP (n=243) | R-CHOP (n=244) |
|---|---|---|
| Note: All results are based on the analysis performed at a median follow-up duration of 40 months except for the overall survival analysis, which was performed at a median follow-up of 78.5 months. CI = Confidence Interval; IPI = International Prognostic Index; LDH = Lactate dehydrogenase | ||
| ||
| Progression-Free Survival (by independent radiographic assessment) | ||
| Events n (%) | 133 (55) | 165 (68) |
| Median* (months) | 25 | 14 |
| (95% CI) | (20, 32) | (12, 17) |
| Hazard ratio† | 0.63 | |
| (95% CI) | (0.50, 0.79) | |
| p-value‡ | <0.001 | |
| Complete Response Rate (CR)§ | ||
| n (%) | 108 (44) | 82 (34) |
| (95% CI) | (38, 51) | (28, 40) |
| Overall Response Rate (CR + Cru + PR)¶ | ||
| n (%) | 214 (88) | 208 (85) |
| (95% CI) | (83, 92) | (80, 89) |
| Overall Survival | ||
| Events n (%) | 103 (42) | 138 (57) |
| Median* (months) | 91 | 56 |
| (95% CI) | (71, NE) | (47, 69) |
| Hazard ratio† | 0.66 | |
| (95% CI) | (0.51, 0.85) | |
| Figure 5: Progression-Free Survival VcR-CAP vs R-CHOP (previously Untreated Mantle Cell Lymphoma Study) |
 |
Key: R-CHOP = rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone; VcR-CAP = bortezomib, rituximab, cyclophosphamide, doxorubicin, and prednisone.
Figure 6: Overall Survival VcR-CAP vs R-CHOP (previously Untreated Mantle Cell Lymphoma Study)
Key: R-CHOP = rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone; VcR-CAP = bortezomib, rituximab, cyclophosphamide, doxorubicin, and prednisone.
A Phase 2 Single-Arm Clinical Study in Relapsed Mantle Cell Lymphoma after Prior Therapy
The safety and efficacy of bortezomib for injection in relapsed or refractory mantle cell lymphoma were evaluated in an open-label, single-arm, multicenter study (NCT00063713) of 155 patients with progressive disease who had received at least one prior therapy. The median age of the patients was 65 years (42, 89), 81% were male, and 92% were Caucasian. Of the total, 75% had one or more extra-nodal sites of disease, and 77% were Stage 4. In 91% of the patients, prior therapy included all of the following: an anthracycline or mitoxantrone, cyclophosphamide, and rituximab. A total of thirty-seven percent (37%) of patients were refractory to their last prior therapy. An intravenous bolus injection of bortezomib for injection 1.3 mg/m2/dose was administered twice weekly for two weeks on Days 1, 4, 8, and 11 followed by a ten day rest period (Days 12 to 21) for a maximum of 17 treatment cycles. Patients achieving a CR or CRu were treated for four cycles beyond first evidence of CR or CRu. The study employed dose modifications for toxicity [see Dosage and Administration (2.6, 2.7)].
Responses to bortezomib for injection are shown in Table 19. Response rates to bortezomib for injection were determined according to the International Workshop Response Criteria (IWRC) based on independent radiologic review of CT scans. The median number of cycles administered across all patients was four; in responding patients the median number of cycles was eight. The median time to response was 40 days (range: 31 to 204 days). The median duration of follow-up was more than 13 months.
| Response Analyses (N=155) | N (%) | 95% CI |
| Overall Response Rate (IWRC) (CR + CRu + PR) | 48 (31) | (24, 39) |
| Complete Response (CR + CRu) | 12 (8) | (4, 13) |
| CR | 10 (6) | (3, 12) |
| CRu | 2 (1) | (0, 5) |
| Partial Response (PR) | 36 (23) | (17, 31) |
| Duration of Response | Median | 95% CI |
| CR + CRu + PR (N=48) | 9.3 months | (5.4, 13.8) |
| CR + CRu (N=12) | 15.4 months | (13.4, 15.4) |
| PR (N=36) | 6.1 months | (4.2, 9.3) |
Find bortezomib injection 3.5 MG VIAL medical information:
Find bortezomib injection 3.5 MG VIAL medical information:
bortezomib injection 3.5 MG VIAL Quick Finder
Health Professional Information
Clinical Studies
14 CLINICAL STUDIES
14.1 Multiple Myeloma
Randomized, Open-Label Clinical Study in Patients with Previously Untreated Multiple Myeloma
A prospective, international, randomized (1:1), open-label clinical study (NCT00111319) of 682 patients was conducted to determine whether bortezomib for injection administered intravenously (1.3 mg/m2) in combination with melphalan (9 mg/m2) and prednisone (60 mg/m2) resulted in improvement in time to progression (TTP) when compared to melphalan (9 mg/m2) and prednisone (60 mg/m2) in patients with previously untreated multiple myeloma. Treatment was administered for a maximum of nine cycles (approximately 54 weeks) and was discontinued early for disease progression or unacceptable toxicity. Antiviral prophylaxis was recommended for patients on the bortezomib for injection study arm.
The median age of the patients in the study was 71 years (48;91), 50% were male, 88% were Caucasian and the median Karnofsky performance status score for the patients was 80 (60;100). Patients had IgG/IgA/Light chain myeloma in 63%/25%/8% instances, a median hemoglobin of 105 g/L (64;165), and a median platelet count of 221,500/microliter (33,000;587,000).
Efficacy results for the trial are presented in Table 14. At a prespecified interim analysis (with median follow-up of 16.3 months), the combination of bortezomib for injection, melphalan and prednisone therapy resulted in significantly superior results for time to progression, progression-free survival, overall survival and response rate. Further enrollment was halted, and patients receiving melphalan and prednisone were offered bortezomib for injection in addition. A later, prespecified analysis of overall survival (with median follow-up of 36.7 months with a hazard ratio of 0.65, 95% CI: 0.51, 0.84) resulted in a statistically significant survival benefit for the bortezomib for injection, melphalan and prednisone treatment arm despite subsequent therapies including bortezomib for injection based regimens. In an updated analysis of overall survival based on 387 deaths (median follow-up 60.1 months), the median overall survival for the bortezomib for injection, melphalan and prednisone treatment arm was 56.4 months and for the melphalan and prednisone treatment arm was 43.1 months, with a hazard ratio of 0.695 (95% CI: 0.57, 0.85).
| Efficacy Endpoint | Bortezomib for Injection, Melphalan and Prednisone (n=344) | Melphalan and Prednisone (n=338) |
|---|---|---|
| Note: All results are based on the analysis performed at a median follow-up duration of 16.3 months except for the overall survival analysis. | ||
| ||
| Time to Progression | ||
| Events n (%) | 101 (29) | 152 (45) |
| Median* (months) | 20.7 | 15.0 |
| (95% CI) | (17.6, 24.7) | (14.1, 17.9) |
| Hazard ratio † | 0.54 | |
| (95% CI) | (0.42, 0.70) | |
| p-value ‡ | 0.000002 | |
| Progression-Free Survival | ||
| Events n (%) | 135 (39) | 190 (56) |
| Median* (months) | 18.3 | 14.0 |
| (95% CI) | (16.6, 21.7) | (11.1, 15.0) |
| Hazard ratio† | 0.61 | |
| (95% CI) | (0.49, 0.76) | |
| p-value‡ | 0.00001 | |
| Response Rate | ||
| CR§ n (%) | 102 (30) | 12 (4) |
| PR§ n (%) | 136 (40) | 103 (30) |
| nCR n (%) | 5 (1) | 0 |
| CR + PR§ n (%) | 238 (69) | 115 (34) |
| p-value¶ | <10-10 | |
| Overall Survival at Median Follow-Up of 36.7 Months | ||
| Events (deaths) n (%) | 109 (32) | 148 (44) |
| Median* (months) | Not Reached | 43.1 |
| (95% CI) | (46.2, NR) | (34.8, NR) |
| Hazard ratio† | 0.65 | |
| (95% CI) | (0.51, 0.84) | |
| p-value‡ | 0.00084 | |
TTP was statistically significantly longer on the bortezomib for injection, melphalan and prednisone arm (see Figure 1). (median follow-up 16.3 months)
 |
Overall survival was statistically significantly longer on the bortezomib for injection, melphalan and prednisone arm (see Figure 2). (median follow-up 60.1 months)
 |
Randomized, Clinical Study in Relapsed Multiple Myeloma of Bortezomib for Injection vs Dexamethasone
A prospective Phase 3, international, randomized (1:1), stratified, open-label clinical study (NCT00048230) enrolling 669 patients was designed to determine whether bortezomib for injection resulted in improvement in time to progression (TTP) compared to high-dose dexamethasone in patients with progressive multiple myeloma following 1 to 3 prior therapies. Patients considered to be refractory to prior high-dose dexamethasone were excluded as were those with baseline Grade ≥2 peripheral neuropathy or platelet counts <50,000/μL. A total of 627 patients were evaluable for response.
Stratification factors were based on the number of lines of prior therapy the patient had previously received (one previous line vs more than one line of therapy), time of progression relative to prior treatment (progression during or within six months of stopping their most recent therapy vs relapse >6 months after receiving their most recent therapy), and screening beta2-microglobulin levels (≤2.5 mg/L vs >2.5 mg/L). Baseline patient and disease characteristics are summarized in Table 15.
| Patient Characteristics | Bortezomib for Injection (N=333) | Dexamethasone (N=336) |
|---|---|---|
| Median age in years (range) | 62.0 (33, 84) | 61.0 (27, 86) |
| Gender: Male/female | 56% / 44% | 60% / 40% |
| Race: Caucasian/black/other | 90% / 6% / 4% | 88% / 7% / 5% |
| Karnofsky performance status score ≤70 | 13% | 17% |
| Hemoglobin <100 g/L | 32% | 28% |
| Platelet count <75 × 109/L | 6% | 4% |
| Disease Characteristics | ||
| Type of myeloma (%): IgG/IgA/Light chain | 60% / 23% / 12% | 59% / 24% / 13% |
| Median beta2-microglobulin (mg/L) | 3.7 | 3.6 |
| Median albumin (g/L) | 39.0 | 39.0 |
| Creatinine clearance ≤30 mL/min [n (%)] | 17 (5%) | 11 (3%) |
| Median Duration of Multiple Myeloma Since Diagnosis (Years) | 3.5 | 3.1 |
| Number of Prior Therapeutic Lines of Treatment | ||
| Median | 2 | 2 |
| 1 prior line | 40% | 35% |
| >1 prior line | 60% | 65% |
| Previous Therapy | ||
| Any prior steroids, e.g., dexamethasone, VAD | 98% | 99% |
| Any prior anthracyclines, e.g., VAD, mitoxantrone | 77% | 76% |
| Any prior alkylating agents, e.g., MP, VBMCP | 91% | 92% |
| Any prior thalidomide therapy | 48% | 50% |
| Vinca alkaloids | 74% | 72% |
| Prior stem cell transplant/other high-dose therapy | 67% | 68% |
| Prior experimental or other types of therapy | 3% | 2% |
Patients in the bortezomib for injection treatment group were to receive 8, three week treatment cycles followed by 3, five week treatment cycles of bortezomib for injection. Patients achieving a CR were treated for four cycles beyond first evidence of CR. Within each three week treatment cycle, bortezomib for injection 1.3 mg/m2/dose alone was administered by intravenous bolus twice weekly for two weeks on Days 1, 4, 8, and 11 followed by a ten day rest period (Days 12 to 21). Within each five week treatment cycle, bortezomib for injection 1.3 mg/m2/dose alone was administered by intravenous bolus once weekly for four weeks on Days 1, 8, 15, and 22 followed by a 13 day rest period (Days 23 to 35) [see Dosage and Administration (2.2)].
Patients in the dexamethasone treatment group were to receive 4, five week treatment cycles followed by 5, four week treatment cycles. Within each five week treatment cycle, dexamethasone 40 mg/day PO was administered once daily on Days 1 to 4, 9 to 12, and 17 to 20 followed by a 15 day rest period (Days 21 to 35). Within each four week treatment cycle, dexamethasone 40 mg/day PO was administered once daily on Days 1 to 4 followed by a 24 day rest period (Days 5 to 28). Patients with documented progressive disease on dexamethasone were offered bortezomib for injection at a standard dose and schedule on a companion study. Following a preplanned interim analysis of time to progression, the dexamethasone arm was halted and all patients randomized to dexamethasone were offered bortezomib for injection, regardless of disease status.
In the bortezomib for injection arm, 34% of patients received at least one bortezomib for injection dose in all eight of the three week cycles of therapy, and 13% received at least one dose in all 11 cycles. The average number of bortezomib for injection doses during the study was 22, with a range of 1 to 44. In the dexamethasone arm, 40% of patients received at least one dose in all four of the five week treatment cycles of therapy, and 6% received at least one dose in all nine cycles.
The time to event analyses and response rates from the relapsed multiple myeloma study are presented in Table 16. Response and progression were assessed using the European Group for Blood and Marrow Transplantation (EBMT) criteria. Complete response (CR) required <5% plasma cells in the marrow, 100% reduction in M-protein, and a negative immunofixation test (IF-). Partial response (PR) requires ≥50% reduction in serum myeloma protein and ≥90% reduction of urine myeloma protein on at least two occasions for a minimum of at least six weeks along with stable bone disease and normal calcium. Near complete response (nCR) was defined as meeting all the criteria for complete response including 100% reduction in M-protein by protein electrophoresis; however, M-protein was still detectable by immunofixation (IF+).
| Efficacy Endpoint | All Patients | 1 Prior Line of Therapy | >1 Prior Line of Therapy | |||
|---|---|---|---|---|---|---|
| Bortezomib | Dex | Bortezomib | Dex | Bortezomib | Dex | |
| (n=333) | (n=336) | (n=132) | (n=119) | (n=200) | (n=217) | |
| ||||||
| Time to Progression Events n (%) | 147 (44) | 196 (58) | 55 (42) | 64 (54) | 92 (46) | 132 (61) |
| Median* (95% CI) | 6.2 mo (4.9, 6.9) | 3.5 mo (2.9, 4.2) | 7.0 mo (6.2, 8.8) | 5.6 mo (3.4, 6.3) | 4.9 mo (4.2, 6.3) | 2.9 mo (2.8, 3.5) |
| Hazard ratio† (95% CI) | 0.55 (0.44, 0.69) | 0.55 (0.38, 0.81) | 0.54 (0.41, 0.72) | |||
| p-value‡ | <0.0001 | 0.0019 | <0.0001 | |||
| Overall Survival Events (deaths) n (%) | 51 (15) | 84 (25) | 12 (9) | 24 (20) | 39 (20) | 60 (28) |
| Hazard ratio† (95% CI) | 0.57 (0.40, 0.81) | 0.39 (0.19, 0.81) | 0.65 (0.43, 0.97) | |||
| p-value‡, § | <0.05 | <0.05 | <0.05 | |||
| Response Rate Population¶ n=627 | n=315 | n=312 | n=128 | n=110 | n=187 | n=202 |
| CR# n (%) | 20 (6) | 2 (<1) | 8 (6) | 2 (2) | 12 (6) | 0 (0) |
| PR# n (%) | 101 (32) | 54 (17) | 49 (38) | 27 (25) | 52 (28) | 27 (13) |
| nCR#,Þ n (%) | 21 (7) | 3 (<1) | 8 (6) | 2 (2) | 13 (7) | 1 (<1) |
| CR + PR# n (%) | 121 (38) | 56 (18) | 57 (45) | 29 (26) | 64 (34) | 27 (13) |
| p-valueß | <0.0001 | 0.0035 | <0.0001 | |||
TTP was statistically significantly longer on the bortezomib for injection arm (see Figure 3).
 |
As shown in Figure 4, bortezomib for injection had a significant survival advantage relative to dexamethasone (p <0.05). The median follow-up was 8.3 months.
 |
For the 121 patients achieving a response (CR or PR) on the bortezomib for injection arm, the median duration was 8.0 months (95% CI: 6.9, 11.5 months) compared to 5.6 months (95% CI: 4.8, 9.2 months) for the 56 responders on the dexamethasone arm. The response rate was significantly higher on the bortezomib for injection arm regardless of beta2-microglobulin levels at baseline.
Randomized, Open-Label Clinical Study of Bortezomib for Injection Subcutaneous vs Intravenous in Relapsed Multiple Myeloma
An open-label, randomized, Phase 3 noninferiority study (NCT00722566) compared the efficacy and safety of the subcutaneous administration of bortezomib for injection vs the intravenous administration. This study included 222 bortezomib naïve patients with relapsed multiple myeloma, who were randomized in a 2:1 ratio to receive 1.3 mg/m2 of bortezomib for injection by either the subcutaneous (n=148) or intravenous (n=74) route for eight cycles. Patients who did not obtain an optimal response (less than Complete Response (CR)) to therapy with bortezomib for injection alone after four cycles were allowed to receive oral dexamethasone 20 mg daily on the day of and after bortezomib for injection administration (82 patients in subcutaneous treatment group and 39 patients in the intravenous treatment group). Patients with baseline Grade ≥2 peripheral neuropathy or neuropathic pain, or platelet counts <50,000/μL were excluded. A total of 218 patients were evaluable for response.
Stratification factors were based on the number of lines of prior therapy the patient had received (one previous line vs more than one line of therapy), and international staging system (ISS) stage (incorporating beta2-microglobulin and albumin levels; Stages I, II, or III).
The baseline demographic and other characteristics of the two treatment groups are summarized as follows: the median age of the patient population was approximately 64 years of age (range: 38 to 88 years), primarily male (subcutaneous: 50%, intravenous: 64%); the primary type of myeloma is IgG (subcutaneous: 65% IgG, 26% IgA, 8% light chain; intravenous: 72% IgG, 19% IgA, 8% light chain), ISS staging I/II/III (%) was 27, 41, 32 for both subcutaneous and intravenous, Karnofsky performance status score was ≤70% in 22% of subcutaneous and 16% of intravenous, creatinine clearance was 67.5 mL/min in subcutaneous and 73 mL/min in intravenous, the median years from diagnosis was 2.68 and 2.93 in subcutaneous and intravenous respectively and the proportion of patients with more than one prior line of therapy was 38% in subcutaneous and 35% in intravenous.
This study met its primary (noninferiority) objective that single agent subcutaneous bortezomib for injection retains at least 60% of the overall response rate after four cycles relative to single agent intravenous bortezomib for injection. The results are provided in Table 17.
| Subcutaneous Bortezomib | Intravenous Bortezomib | |
|---|---|---|
| Intent to Treat Population | (n=148) | (n=74) |
| ||
| Primary Endpoint | ||
| Response Rate at 4 Cycles | ||
| ORR (CR + PR) n(%) | 63 (43) | 31 (42) |
| Ratio of Response Rates (95% CI) | 1.01 (0.73, 1.40) | |
| CR n (%) | 11 (7) | 6 (8) |
| PR n (%) | 52 (35) | 25 (34) |
| nCR n (%) | 9 (6) | 4 (5) |
| Secondary Endpoints | ||
| Response Rate at 8 Cycles | ||
| ORR (CR + PR) | 78 (53) | 38 (51) |
| CR n (%) | 17 (11) | 9 (12) |
| PR n (%) | 61 (41) | 29 (39) |
| nCR n (%) | 14 (9) | 7 (9) |
| Median Time to Progression, months | 10.4 | 9.4 |
| Median Progression-Free Survival, months | 10.2 | 8.0 |
| 1 Year Overall Survival (%)* | 72.6 | 76.7 |
A Randomized, Phase 2 Dose-Response Study in Relapsed Multiple Myeloma
An open-label, multicenter study randomized 54 patients with multiple myeloma who had progressed or relapsed on or after front-line therapy to receive bortezomib for injection 1 mg/m2 or 1.3 mg/m2 intravenous bolus twice weekly for two weeks on Days 1, 4, 8, and 11 followed by a ten day rest period (Days 12 to 21). The median duration of time between diagnosis of multiple myeloma and first dose of bortezomib for injection on this trial was two years, and patients had received a median of one prior line of treatment (median of three prior therapies). A single complete response was seen at each dose. The overall response rates (CR + PR) were 30% (8/27) at 1 mg/m2 and 38% (10/26) at 1.3 mg/m2 .
A Phase 2 Open-Label Extension Study in Relapsed Multiple Myeloma
Patients from the two Phase 2 studies, who in the investigators' opinion would experience additional clinical benefit, continued to receive bortezomib for injection beyond 8 cycles on an extension study. Sixty-three (63) patients from the Phase 2 multiple myeloma studies were enrolled and received a median of seven additional cycles of bortezomib for injection therapy for a total median of 14 cycles (range: 7 to 32). The overall median dosing intensity was the same in both the parent protocol and extension study. Sixty-seven percent (67%) of patients initiated the extension study at the same or higher dose intensity at which they completed the parent protocol, and 89% of patients maintained the standard three week dosing schedule during the extension study. No new cumulative or new long-term toxicities were observed with prolonged bortezomib for injection treatment [see Adverse Reactions (6.1)].
A Single-Arm Trial of Retreatment in Relapsed Multiple Myeloma
A single-arm, open-label trial (NCT00431769) was conducted to determine the efficacy and safety of retreatment with bortezomib for injection. One hundred and thirty patients (≥18 years of age) with multiple myeloma who previously had at least partial response on a bortezomib for injection-containing regimen (median of two prior lines of therapy [range: 1–7]) were retreated upon progression with bortezomib for injection administered intravenously. Patients were excluded from trial participation if they had peripheral neuropathy or neuropathic pain of Grade ≥2. At least six months after prior bortezomib for injection therapy, bortezomib for injection was restarted at the last tolerated dose of 1.3 mg/m2 (n=93) or ≤1.0 mg/m2 (n=37) and given on Days 1, 4, 8 and 11 every three weeks for maximum of eight cycles either as single agent or in combination with dexamethasone in accordance with the standard of care. Dexamethasone was administered in combination with bortezomib for injection to 83 patients in Cycle 1 with an additional 11 patients receiving dexamethasone during the course of bortezomib for injection retreatment cycles.
The primary endpoint was best confirmed response to retreatment as assessed by European Group for Blood and Marrow Transplantation (EBMT) criteria. Fifty of the 130 patients achieved a best confirmed response of Partial Response or better for an overall response rate of 38.5% (95% CI: 30.1, 47.4). One patient achieved a Complete Response and 49 achieved Partial Response. In the 50 responding patients, the median duration of response was 6.5 months and the range was 0.6 to 19.3 months.
14.2 Mantle Cell Lymphoma
A Randomized, Open-Label Clinical Study in Patients with Previously Untreated Mantle Cell Lymphoma
A randomized, open-label, Phase 3 study (NCT00722137) was conducted in 487 adult patients with previously untreated mantle cell lymphoma (Stage II, III or IV) who were ineligible or not considered for bone marrow transplantation to determine whether bortezomib administered in combination with rituximab, cyclophosphamide, doxorubicin, and prednisone (VcR-CAP) resulted in improvement in progression-free survival (PFS) when compared to the combination of rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP). This clinical study utilized independent pathology confirmation and independent radiologic response assessment.
Patients in the VcR-CAP treatment arm received bortezomib (1.3 mg/m2) administered intravenously on Days 1, 4, 8, and 11 (rest period Days 12 to 21); rituximab (375 mg/m2) on Day 1; cyclophosphamide (750 mg/m2) on Day 1; doxorubicin (50 mg/m2) on Day 1; and prednisone (100 mg/m2) on Day 1 through Day 5 of the 21 day treatment cycle. For patients with a response first documented at Cycle 6, two additional treatment cycles were allowed.
Median patient age was 66 years, 74% were male, 66% were Caucasian and 32% were Asian. Sixty-nine percent of patients had a positive bone marrow aspirate and/or a positive bone marrow biopsy for MCL, 54% of patients had an International Prognostic Index (IPI) score of three (high-intermediate) or higher and 76% had Stage IV disease.
The majority of the patients in both groups received six or more cycles of treatment, 84% in the VcR-CAP group and 83% in the R-CHOP group. Median number of cycles received by patients in both treatment arms was six with 17% of patients in the R-CHOP group and 14% of subjects in the VcR-CAP group receiving up to two additional cycles.
The efficacy results for PFS, CR and ORR with a median follow-up of 40 months are presented in Table 18. The response criteria used to assess efficacy were based on the International Workshop to Standardize Response Criteria for Non-Hodgkin's Lymphoma (IWRC). Final overall survival results at a median follow-up of 78.5 months are also presented in Table 18 and Figure 6. The combination of VcR-CAP resulted in statistically significant prolongation of PFS compared with R-CHOP (see Table 18, Figure 5).
| Efficacy Endpoint n: Intent to Treat patients | VcR-CAP (n=243) | R-CHOP (n=244) |
|---|---|---|
| Note: All results are based on the analysis performed at a median follow-up duration of 40 months except for the overall survival analysis, which was performed at a median follow-up of 78.5 months. CI = Confidence Interval; IPI = International Prognostic Index; LDH = Lactate dehydrogenase | ||
| ||
| Progression-Free Survival (by independent radiographic assessment) | ||
| Events n (%) | 133 (55) | 165 (68) |
| Median* (months) | 25 | 14 |
| (95% CI) | (20, 32) | (12, 17) |
| Hazard ratio† | 0.63 | |
| (95% CI) | (0.50, 0.79) | |
| p-value‡ | <0.001 | |
| Complete Response Rate (CR)§ | ||
| n (%) | 108 (44) | 82 (34) |
| (95% CI) | (38, 51) | (28, 40) |
| Overall Response Rate (CR + Cru + PR)¶ | ||
| n (%) | 214 (88) | 208 (85) |
| (95% CI) | (83, 92) | (80, 89) |
| Overall Survival | ||
| Events n (%) | 103 (42) | 138 (57) |
| Median* (months) | 91 | 56 |
| (95% CI) | (71, NE) | (47, 69) |
| Hazard ratio† | 0.66 | |
| (95% CI) | (0.51, 0.85) | |
| Figure 5: Progression-Free Survival VcR-CAP vs R-CHOP (previously Untreated Mantle Cell Lymphoma Study) |
 |
Key: R-CHOP = rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone; VcR-CAP = bortezomib, rituximab, cyclophosphamide, doxorubicin, and prednisone.
Figure 6: Overall Survival VcR-CAP vs R-CHOP (previously Untreated Mantle Cell Lymphoma Study)
Key: R-CHOP = rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone; VcR-CAP = bortezomib, rituximab, cyclophosphamide, doxorubicin, and prednisone.
A Phase 2 Single-Arm Clinical Study in Relapsed Mantle Cell Lymphoma after Prior Therapy
The safety and efficacy of bortezomib for injection in relapsed or refractory mantle cell lymphoma were evaluated in an open-label, single-arm, multicenter study (NCT00063713) of 155 patients with progressive disease who had received at least one prior therapy. The median age of the patients was 65 years (42, 89), 81% were male, and 92% were Caucasian. Of the total, 75% had one or more extra-nodal sites of disease, and 77% were Stage 4. In 91% of the patients, prior therapy included all of the following: an anthracycline or mitoxantrone, cyclophosphamide, and rituximab. A total of thirty-seven percent (37%) of patients were refractory to their last prior therapy. An intravenous bolus injection of bortezomib for injection 1.3 mg/m2/dose was administered twice weekly for two weeks on Days 1, 4, 8, and 11 followed by a ten day rest period (Days 12 to 21) for a maximum of 17 treatment cycles. Patients achieving a CR or CRu were treated for four cycles beyond first evidence of CR or CRu. The study employed dose modifications for toxicity [see Dosage and Administration (2.6, 2.7)].
Responses to bortezomib for injection are shown in Table 19. Response rates to bortezomib for injection were determined according to the International Workshop Response Criteria (IWRC) based on independent radiologic review of CT scans. The median number of cycles administered across all patients was four; in responding patients the median number of cycles was eight. The median time to response was 40 days (range: 31 to 204 days). The median duration of follow-up was more than 13 months.
| Response Analyses (N=155) | N (%) | 95% CI |
| Overall Response Rate (IWRC) (CR + CRu + PR) | 48 (31) | (24, 39) |
| Complete Response (CR + CRu) | 12 (8) | (4, 13) |
| CR | 10 (6) | (3, 12) |
| CRu | 2 (1) | (0, 5) |
| Partial Response (PR) | 36 (23) | (17, 31) |
| Duration of Response | Median | 95% CI |
| CR + CRu + PR (N=48) | 9.3 months | (5.4, 13.8) |
| CR + CRu (N=12) | 15.4 months | (13.4, 15.4) |
| PR (N=36) | 6.1 months | (4.2, 9.3) |
Health Professional Information
{{section_name_patient}}
{{section_body_html_patient}}
Resources
Didn’t find what you were looking for? Contact us.
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine.
*Speak with a Pfizer Medical Information Professional regarding your medical inquiry. Available 9AM-5Pm ET Monday to Friday; excluding holidays.
Submit a medical question for Pfizer prescription products.
Report Adverse Event
To report an adverse event related to the Pfizer-BioNTech COVID-19 Vaccine, and you are not part of a clinical trial* for this product, click the link below to submit your information:
Pfizer Safety Reporting Site*If you are involved in a clinical trial for this product, adverse events should be reported to your coordinating study site.
If you cannot use the above website, or would like to report an adverse event related to a different Pfizer product, please call Pfizer Safety at (800) 438-1985.
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or call (800) 822-7967.