BICILLIN® C-R
(penicillin G benzathine, penicillin G procaine)
Find BICILLIN® C-R medical information:
Find BICILLIN® C-R medical information:
BICILLIN® C-R Quick Finder
Boxed Warning
WARNING: NOT FOR INTRAVENOUS USE. DO NOT INJECT INTRAVENOUSLY OR ADMIX WITH OTHER INTRAVENOUS SOLUTIONS. THERE HAVE BEEN REPORTS OF INADVERTENT INTRAVENOUS ADMINISTRATION OF PENICILLIN G BENZATHINE WHICH HAS BEEN ASSOCIATED WITH CARDIORESPIRATORY ARREST AND DEATH. Prior to administration of this drug, carefully read theWARNINGS,ADVERSE REACTIONS, andDOSAGE AND ADMINISTRATIONsections of the labeling.
Indications and Usage
INDICATIONS AND USAGE
To reduce the development of drug-resistant bacteria and maintain the effectiveness of Bicillin C-R and other antibacterial drugs, Bicillin C-R should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
This drug is indicated in the treatment of moderately severe infections due to penicillin-G-susceptible microorganisms that are susceptible to serum levels common to this particular dosage form. Therapy should be guided by bacteriological studies (including susceptibility testing) and by clinical response.
Bicillin C-R is indicated in the treatment of the following in adults and pediatric patients:
Moderately severe to severe infections of the upper-respiratory tract, scarlet fever, erysipelas, and skin and soft-tissue infections due to susceptible streptococci.
NOTE: Streptococci in Groups A, C, G, H, L, and M are very sensitive to penicillin G. Other groups, including Group D (enterococci), are resistant. Penicillin G sodium or potassium is recommended for streptococcal infections with bacteremia.
Moderately severe pneumonia and otitis media due to susceptible Streptococcus pneumoniae.
NOTE: Severe pneumonia, empyema, bacteremia, pericarditis, meningitis, peritonitis, and arthritis of pneumococcal etiology are better treated with penicillin G sodium or potassium during the acute stage.
When high, sustained serum levels are required, penicillin G sodium or potassium, either IM or IV, should be used. This drug should not be used in the treatment of venereal diseases, including syphilis, gonorrhea, yaws, bejel, and pinta.
Dosage and Administration
DOSAGE AND ADMINISTRATION
Streptococcal Infections Group A
Infections of the upper-respiratory tract, skin and soft-tissue infections, scarlet fever, and erysipelas.
The following doses are recommended:
Adults and pediatric patients over 60 lbs. in weight: 2,400,000 units.
Pediatric patients from 30 to 60 lbs.: 900,000 units to 1,200,000 units.
Pediatric patients under 30 lbs.: 600,000 units.
NOTE: Treatment with the recommended dosage is usually given at a single session using multiple IM sites when indicated. An alternative dosage schedule may be used, giving one-half (1/2) the total dose on day 1 and one-half (1/2) on day 3. This will also insure the penicillinemia required over a 10-day period; however, this alternate schedule should be used only when the physician can be assured of the patient's cooperation.
Pneumococcal Infections (except pneumococcal meningitis)
600,000 units in pediatric patients and 1,200,000 units in adults, repeated every 2 or 3 days until the temperature is normal for 48 hours. Other forms of penicillin may be necessary for severe cases.
Method of Administration
Bicillin C-R is intended for Intramuscular Injection ONLY. Do not inject into or near an artery or nerve, or intravenously or admix with other intravenous solutions. (See WARNINGS section).
Administer by DEEP INTRAMUSCULAR INJECTION in the upper, outer quadrant of the buttock (dorsogluteal) or the ventrogluteal site. In neonates, infants and small children, the midlateral aspect of the thigh may be preferable. Administration in the anterolateral thigh is not recommended due to the adverse effects observed (see WARNINGS section), and vascularity of this region. When doses are repeated, vary the injection site.
Because of the high concentration of suspended material in this product, the needle may be blocked if the injection is not made at a slow, steady rate.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit.
Contraindications
Warnings and Precautions
WARNINGS
WARNING: NOT FOR INTRAVENOUS USE. DO NOT INJECT INTRAVENOUSLY OR ADMIX WITH OTHER INTRAVENOUS SOLUTIONS. THERE HAVE BEEN REPORTS OF INADVERTENT INTRAVENOUS ADMINISTRATION OF PENICILLIN G BENZATHINE WHICH HAS BEEN ASSOCIATED WITH CARDIORESPIRATORY ARREST AND DEATH. Prior to administration of this drug, carefully read the WARNINGS, ADVERSE REACTIONS, and DOSAGE AND ADMINISTRATION sections of the labeling. |
The combination of penicillin G benzathine and penicillin G procaine should only be prescribed for the indications listed in this insert.
Anaphylaxis
SERIOUS AND OCCASIONALLY FATAL HYPERSENSITIVITY (ANAPHYLACTIC) REACTIONS HAVE BEEN REPORTED IN PATIENTS ON PENICILLIN THERAPY. THESE REACTIONS ARE MORE LIKELY TO OCCUR IN INDIVIDUALS WITH A HISTORY OF PENICILLIN HYPERSENSITIVITY AND/OR A HISTORY OF SENSITIVITY TO MULTIPLE ALLERGENS. THERE HAVE BEEN REPORTS OF INDIVIDUALS WITH A HISTORY OF PENICILLIN HYPERSENSITIVITY WHO HAVE EXPERIENCED SEVERE REACTIONS WHEN TREATED WITH CEPHALOSPORINS. BEFORE INITIATING THERAPY WITH BICILLIN C-R CAREFUL INQUIRY SHOULD BE MADE CONCERNING PREVIOUS HYPERSENSITIVITY REACTIONS TO PENICILLINS, CEPHALOSPORINS OR OTHER ALLERGENS. IF AN ALLERGIC REACTION OCCURS, BICILLIN C-R SHOULD BE DISCONTINUED AND APPROPRIATE THERAPY INSTITUTED. SERIOUS ANAPHYLACTIC REACTIONS REQUIRE IMMEDIATE EMERGENCY TREATMENT WITH EPINEPHRINE. OXYGEN, INTRAVENOUS STEROIDS AND AIRWAY MANAGEMENT, INCLUDING INTUBATION, SHOULD ALSO BE ADMINISTERED AS INDICATED.
Severe cutaneous adverse reactions
Severe cutaneous adverse reactions (SCAR), such as Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), drug reaction with eosinophilia and systemic symptoms (DRESS), and acute generalized exanthematous pustulosis (AGEP) have been reported in patients taking penicillin G (the active moiety in Bicillin C-R). When SCAR is suspected, Bicillin C-R should be discontinued immediately and an alternative treatment should be considered.
Methemoglobinemia
Cases of methemoglobinemia have been reported in association with local anesthetic use. Although all patients are at risk for methemoglobinemia, patients with glucose-6-phosphate dehydrogenase deficiency, congenital or idiopathic methemoglobinemia, cardiac or pulmonary compromise, infants under 6 months of age, and concurrent exposure to oxidizing agents or their metabolites are more susceptible to developing clinical manifestations of the condition. If local anesthetics must be used in these patients, close monitoring for symptoms and signs of methemoglobinemia is recommended.
Signs of methemoglobinemia may occur immediately or may be delayed some hours after exposure, and are characterized by a cyanotic skin discoloration and/or abnormal coloration of the blood. Methemoglobin levels may continue to rise; therefore, immediate treatment is required to avert more serious central nervous system (CNS) and cardiovascular adverse effects, including seizures, coma, arrhythmias, and death. Discontinue Bicillin C-R and any other oxidizing agents. Depending on the severity of the signs and symptoms, patients may respond to supportive care, i.e., oxygen therapy, hydration. A more severe clinical presentation may require treatment with methylene blue, exchange transfusion, or hyperbaric oxygen.
Clostridioides difficile associated diarrhea
Clostridioides difficile associated with diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including Bicillin C-R, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
Method of Administration
Do not inject into or near an artery or nerve. See administration instructions below.
Injection into or near a nerve may result in permanent neurological damage.
Inadvertent intravascular administration, including inadvertent direct intra-arterial injection or injection immediately adjacent to arteries, of Bicillin C-R and other penicillin preparations has resulted in severe neurovascular damage, including transverse myelitis with permanent paralysis, gangrene requiring amputation of digits and more proximal portions of extremities, and necrosis and sloughing at and surrounding the injection site consistent with the diagnosis of Nicolau syndrome. Such severe effects have been reported following injections into the buttock, thigh, and deltoid areas. Other serious complications of suspected intravascular administration which have been reported include immediate pallor, mottling, or cyanosis of the extremity both distal and proximal to the injection site, followed by bleb formation; severe edema requiring anterior and/or posterior compartment fasciotomy in the lower extremity. The above-described severe effects and complications have most often occurred in infants and small children. Prompt consultation with an appropriate specialist is indicated if any evidence of compromise of the blood supply occurs at, proximal to, or distal to the site of injection.1–9 (See PRECAUTIONS, and DOSAGE AND ADMINISTRATION sections.)
FOR DEEP INTRAMUSCULAR INJECTION ONLY. There have been reports of inadvertent intravenous administration of penicillin G benzathine which has been associated with cardiorespiratory arrest and death. Therefore, do not inject intravenously or admix with other intravenous solutions. (See DOSAGE AND ADMINISTRATION section.)
Administer by DEEP INTRAMUSCULAR INJECTION ONLY in the upper, outer quadrant of the buttock (dorsogluteal) or the ventrogluteal site. Quadriceps femoris fibrosis and atrophy have been reported following repeated intramuscular injections of penicillin preparations into the anterolateral thigh. Because of these adverse effects and the vascularity of this region, administration in the anterolateral thigh is not recommended.
PRECAUTIONS
General
Prescribing Bicillin C-R in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of a development of drug-resistant bacteria.
Penicillin should be used with caution in individuals with histories of significant allergies and/or asthma.
Care should be taken to avoid intravenous or intra-arterial administration, or injection into or near major peripheral nerves or blood vessels, since such injections may produce neurovascular damage. (See WARNINGS, and DOSAGE AND ADMINISTRATION sections.)
A small percentage of patients are sensitive to procaine. If there is a history of sensitivity, make the usual test: Inject intradermally 0.1 mL of a 1 to 2 percent procaine solution. Development of an erythema, wheal, flare, or eruption indicates procaine sensitivity. Sensitivity should be treated by the usual methods, including barbiturates, and procaine penicillin preparations should not be used. Antihistamines appear beneficial in treatment of procaine reactions.
The use of antibiotics may result in overgrowth of nonsusceptible organisms. Constant observation of the patient is essential. If new infections due to bacteria or fungi appear during therapy, the drug should be discontinued and appropriate measures taken.
Whenever allergic reactions occur, penicillin should be withdrawn unless, in the opinion of the physician, the condition being treated is life-threatening and amenable only to penicillin therapy.
In prolonged therapy with penicillin, and particularly with high-dosage schedules, periodic evaluation of the renal and hematopoietic systems is recommended.
Information for Patients
Inform patients that use of local anesthetics may cause methemoglobinemia, a serious condition that must be treated promptly. Advise patients or caregivers to seek immediate medical attention if they or someone in their care experience the following signs or symptoms: pale, gray, or blue colored skin (cyanosis); headache; rapid heart rate; shortness of breath; lightheadedness; or fatigue.
Diarrhea is a common problem caused by antibiotics which usually ends when the antibiotic is discontinued. Sometimes after starting treatment with antibiotics, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two months after having taken the last dose of the antibiotic. If this occurs, patients should contact their physician as soon as possible.
Patients should be counseled that antibacterial drugs including Bicillin C-R should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When Bicillin C-R is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by Bicillin C-R or other antibacterial drugs in the future.
Laboratory Tests
In streptococcal infections, therapy must be sufficient to eliminate the organism; otherwise, the sequelae of streptococcal disease may occur. Cultures should be taken following completion of treatment to determine whether streptococci have been eradicated.
Drug Interactions
Tetracycline, a bacteriostatic antibiotic, may antagonize the bactericidal effect of penicillin, and concurrent use of these drugs should be avoided.
Concurrent administration of penicillin and probenecid increases and prolongs serum penicillin levels by decreasing the apparent volume of distribution and slowing the rate of excretion by competitively inhibiting renal tubular secretion of penicillin.
Patients who are administered local anesthetics are at increased risk of developing methemoglobinemia when concurrently exposed to the following drugs, which could include other local anesthetics:
| Class | Examples |
|---|---|
Nitrates/Nitrites | nitroglycerin, nitroprusside, nitric oxide, nitrous oxide |
Local anesthetics | articaine, benzocaine, bupivacaine, lidocaine, mepivacaine, prilocaine, procaine, ropivacaine, tetracaine |
Antineoplastic agents | cyclophosphamide, flutamide, hydroxyurea, ifosfamide, rasburicase |
Antibiotics | dapsone, nitrofurantoin, para-aminosalicylic acid, sulfonamides |
Antimalarials | chloroquine, primaquine |
Anticonvulsants | phenobarbital, sodium valproate |
Other drugs | acetaminophen, metoclopramide, quinine, sulfasalazine |
Pregnancy
Teratogenic effects
Reproduction studies performed in the mouse, rat, and rabbit have revealed no evidence of impaired fertility or harm to the fetus due to penicillin G. Human experience with the penicillins during pregnancy has not shown any positive evidence of adverse effects on the fetus. There are, however, no adequate and well-controlled studies in pregnant women showing conclusively that harmful effects of these drugs on the fetus can be excluded. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Nursing Mothers
Soluble penicillin G (the hydrolysate of penicillin G benzathine) is excreted in breast milk. Caution should be exercised when penicillin G benzathine and penicillin G procaine are administered to a nursing woman.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No long-term animal studies have been conducted with these drugs.
Geriatric use
Clinical studies of penicillin G benzathine and penicillin G procaine did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy. This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function (See CLINICAL PHARMACOLOGY.) Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
Adverse Reactions
ADVERSE REACTIONS
As with other penicillins, untoward reactions of the sensitivity phenomena are likely to occur, particularly in individuals who have previously demonstrated hypersensitivity to penicillins or in those with a history of allergy, asthma, hay fever, or urticaria.
The following adverse reactions have been reported with Bicillin C-R during post-marketing experience:
Skin and Appendages: Stevens-Johnson syndrome (SJS) and drug reaction with eosinophilia and systemic symptoms (DRESS) (See WARNINGS.)
The following have been reported with parenteral penicillin G (the active moiety in Bicillin C-R):
General: Hypersensitivity reactions including the following: skin eruptions (maculopapular to exfoliative dermatitis), urticaria, laryngeal edema, fever, eosinophilia; other serum sickness-like reactions (including chills, fever, edema, arthralgia, and prostration); and anaphylaxis including shock and death: severe cutaneous adverse reactions (SCAR), such as toxic epidermal necrolysis (TEN) and acute generalized exanthematous pustulosis (AGEP) (See WARNINGS.) Note: Urticaria, other skin rashes, and serum sickness-like reactions may be controlled with antihistamines and, if necessary, systemic corticosteroids. Whenever such reactions occur, penicillin G should be discontinued unless, in the opinion of the physician, the condition being treated is life-threatening and amenable only to therapy with penicillin G. Serious anaphylactic reactions require immediate emergency treatment with epinephrine. Oxygen, intravenous steroids, and airway management, including intubation, should also be administered as indicated.
Gastrointestinal: Pseudomembranous colitis. Onset of pseudomembranous colitis symptoms may occur during or after antibacterial treatment. (See WARNINGS section.)
Hematologic: Hemolytic anemia, leukopenia, thrombocytopenia.
Neurologic: Neuropathy.
Urogenital: Nephropathy.
The following adverse events have been temporally associated with parenteral administrations of penicillin G benzathine (a component of Bicillin C-R):
Body as a Whole: Hypersensitivity reactions including allergic vasculitis, pruritis, fatigue, asthenia, and pain; aggravation of existing disorder; headache, Nicolau syndrome.
Cardiovascular: Cardiac arrest; hypotension; tachycardia; palpitations; pulmonary hypertension; pulmonary embolism; vasodilation; vasovagal reaction; cerebrovascular accident; syncope.
Gastrointestinal: Nausea, vomiting; blood in stool; intestinal necrosis.
Hemic and Lymphatic: Lymphadenopathy.
Immune System Disorders: Acute myocardial ischemia with or without myocardial infarction may occur as part of an allergic reaction (Kounis syndrome).
Injection Site: Injection site reactions including pain, inflammation, lump, abscess, necrosis, edema, hemorrhage, cellulitis, hypersensitivity, atrophy, ecchymosis, and skin ulcer. Neurovascular reactions including warmth, vasospasm, pallor, mottling, gangrene, numbness of the extremities, cyanosis of the extremities, and neurovascular damage.
Metabolic: Elevated BUN, creatinine, and SGOT.
Musculoskeletal: Joint disorder, periostitis; exacerbation of arthritis; myoglobinuria; rhabdomyolysis.
Nervous System: Nervousness; tremors; dizziness; somnolence; confusion; anxiety; euphoria; transverse myelitis; seizures; coma. A syndrome manifested by a variety of CNS symptoms such as severe agitation with confusion, visual and auditory hallucinations, and a fear of impending death (Hoigne's syndrome), has been reported after administration of penicillin G procaine and, less commonly, after injection of the combination of penicillin G benzathine and penicillin G procaine. Other symptoms associated with this syndrome, such as psychosis, seizures, dizziness, tinnitus, cyanosis, palpitations, tachycardia, and/or abnormal perception in taste, also may occur.
Respiratory: Hypoxia; apnea; dyspnea.
Skin: Diaphoresis.
Special Senses: Blurred vision; blindness.
Urogenital: Neurogenic bladder; hematuria; proteinuria; renal failure; impotence; priapism.
Overdosage
Description
DESCRIPTION
Bicillin C-R (penicillin G benzathine and penicillin G procaine injectable suspension) contains equal amounts of the benzathine and procaine salts of penicillin G. It is available for deep intramuscular injection.
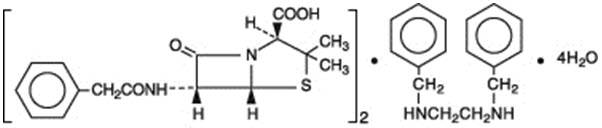
Penicillin G benzathine is prepared by the reaction of dibenzylethylene diamine with two molecules of penicillin G. It is chemically designated as (2S,5R,6R)-3,3-Dimethyl-7-oxo-6-(2-phenylacetamido)-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid compound with N,N'-dibenzylethylenediamine (2:1), tetrahydrate. It occurs as a white, crystalline powder and is very slightly soluble in water and sparingly soluble in alcohol. Its chemical structure is as follows:
Molecular Formula | Molecular Wt. |
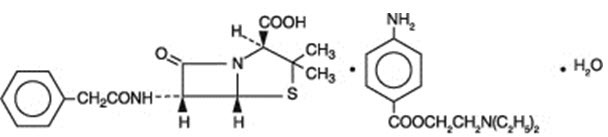
Penicillin G procaine, (2S,5R,6R)-3,3-Dimethyl-7-oxo-6-(2-phenylacetamido)-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid compound with 2-(diethylamino)ethyl p-aminobenzoate (1:1) monohydrate, is an equimolar salt of procaine and penicillin G. It occurs as white crystals or a white, microcrystalline powder and is slightly soluble in water. Its chemical structure is as follows:
Molecular Formula | Molecular Wt. |
Each disposable syringe (2 mL size) contains the equivalent of 1,200,000 units of penicillin G comprising: the equivalent of 600,000 units of penicillin G as the benzathine salt and the equivalent of 600,000 units of penicillin G as the procaine salt in a stabilized aqueous suspension with sodium citrate buffer; and as w/v, approximately 0.5% lecithin, 0.55% carboxymethylcellulose, 0.55% povidone, 0.1% methylparaben, and 0.01% propylparaben.
Bicillin C-R injectable suspension in the disposable-syringe formulation is viscous and opaque. Read CONTRAINDICATIONS, WARNINGS, PRECAUTIONS, and DOSAGE AND ADMINISTRATION sections prior to use.
Clinical Pharmacology
CLINICAL PHARMACOLOGY
General
Penicillin G benzathine and penicillin G procaine have a low solubility and, thus, the drugs are slowly released from intramuscular injection sites. The drugs are hydrolyzed to penicillin G. This combination of hydrolysis and slow absorption results in blood serum levels much lower but more prolonged than other parenteral penicillins.
Intramuscular administration of 600,000 units of Bicillin C-R in adults usually produces peak blood levels of 1.0 to 1.3 units per mL within 3 hours; this level falls to an average concentration of 0.32 units per mL at 12 hours, 0.19 units per mL at 24 hours, and 0.03 units per mL at seven days.
Intramuscular administration of 1,200,000 units of Bicillin C-R in adults usually produces peak blood levels of 2.1 to 2.6 units per mL within 3 hours; this level falls to an average concentration of 0.75 units per mL at 12 hours, 0.28 units per mL at 24 hours, and 0.04 units per mL at seven days.
Approximately 60% of penicillin G is bound to serum protein. The drug is distributed throughout the body tissues in widely varying amounts. Highest levels are found in the kidneys with lesser amounts in the liver, skin, and intestines. Penicillin G penetrates into all other tissues and the spinal fluid to a lesser degree. With normal kidney function, the drug is excreted rapidly by tubular excretion. In neonates and young infants and in individuals with impaired kidney function, excretion is considerably delayed.
Microbiology
Mechanism of Action
Penicillin G exerts a bactericidal action against penicillin-susceptible microorganisms during the stage of active multiplication. It acts through the inhibition of biosynthesis of cell-wall peptidoglycan, rendering the cell wall osmotically unstable resulting in death of the bacterium.
Resistance
Penicillin is not active against penicillinase-producing bacteria, or against organisms resistant to beta-lactams because of alterations in the penicillin-binding proteins. Resistance to penicillin G has not been reported in Streptococcus pyogenes.
Penicillin has been shown to be active against most isolates of the following bacteria, both in vitro and in clinical infections as described in the INDICATIONS AND USAGE section.
Susceptibility Testing
For specific information regarding susceptibility test interpretive criteria and associated test methods and quality control standards recognized by FDA for this drug, please see: https://www.fda.gov/STIC.
References
REFERENCES
- 1.
- SHAW, E.: Transverse myelitis from injection of penicillin. Am. J. Dis. Child., 111:548, 1966.
- 2.
- KNOWLES, J.: Accidental intra-arterial injection of penicillin. Am. J. Dis. Child., 111:552, 1966.
- 3.
- DARBY, C. et al: Ischemia following an intragluteal injection of benzathine-procaine penicillin G mixture in a one-year-old boy. Clin. Pediatrics, 12:485, 1973.
- 4.
- BROWN, L. & NELSON, A.: Postinfectious intravascular thrombosis with gangrene. Arch. Surg., 94:652, 1967.
- 5.
- BORENSTINE, J.: Transverse myelitis and penicillin (Correspondence). Am. J. Dis. Child., 112:166, 1966.
- 6.
- ATKINSON, J.: Transverse myelopathy secondary to penicillin injection. J. Pediatrics, 75:867, 1969.
- 7.
- TALBERT, J. et al: Gangrene of the foot following intramuscular injection in the lateral thigh: A case report with recommendations for prevention. J. Pediatrics, 70:110, 1967.
- 8.
- FISHER, T.: Medicolegal affairs. Canad. Med. Assoc. J., 112:395, 1975.
- 9.
- SCHANZER, H. et al: Accidental intra-arterial injection of penicillin G. JAMA, 242:1289, 1979.
How Supplied/Storage and Handling
HOW SUPPLIED
Bicillin C-R (penicillin G benzathine and penicillin G procaine injectable suspension) is supplied in packages of 10 disposable syringes as follows:
2 mL size, containing 1,200,000 units per syringe (21 gauge, thin-wall 1-inch needle for pediatric use), NDC 60793-601-10.
2 mL size, containing 1,200,000 units per syringe (21 gauge, thin-wall 1-1/2-inch needle), NDC 60793-600-10.
Other
Resources
Didn’t find what you were looking for?
Contact us.
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine.
*Speak with a Pfizer Medical Information Professional regarding your medical inquiry. Available 9AM-5Pm ET Monday to Friday; excluding holidays.
Submit a medical question for Pfizer prescription products.
Report Adverse Event
To report an adverse event related to a Pfizer product and you are not part of a clinical trial* for this product, click the link below to submit your information: Pfizer Safety Reporting Site
*If you are involved in a clinical trial for either product, adverse events should be reported to your coordinating study site.
If you cannot use the above website to report an adverse event related to a Pfizer product, please call Pfizer Medical Information at (800) 438-1985.
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or by calling (800)-332-1088.