DESCRIPTION
Bicillin C-R 900/300 (penicillin G benzathine and penicillin G procaine injectable suspension) contains the equivalent of 900,000 units of penicillin G as the benzathine and 300,000 units of penicillin G as the procaine salts. It is available for deep intramuscular injection.
Penicillin G benzathine is prepared by the reaction of dibenzylethylene diamine with two molecules of penicillin G. It is chemically designated as (2S ,5R,6R)-3,3-Dimethyl-7-oxo-6-(2-phenylacetamido)-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid compound with N,N'-dibenzylethylenediamine (2:1), tetrahydrate. It occurs as a white, crystalline powder and is very slightly soluble in water and sparingly soluble in alcohol. Its chemical structure is as follows:
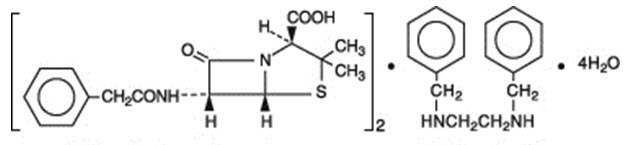 |
| Molecular Formula
(C16H18N2O4S)2∙ C16H20N2∙ 4H2O | Molecular Wt.
981.19 | |
Penicillin G procaine, (2S ,5R,6R)-3,3-Dimethyl-7-oxo-6-(2-phenylacetamido)-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid compound with 2-(diethylamino)ethyl p-aminobenzoate (1:1) monohydrate, is an equimolar salt of procaine and penicillin G. It occurs as white crystals or a white, microcrystalline powder and is slightly soluble in water. Its chemical structure is as follows:
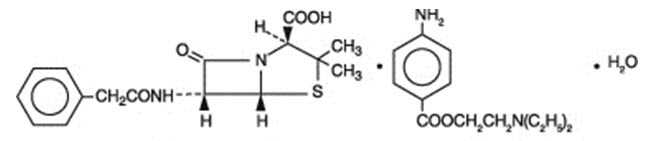 |
| Molecular Formula
C16H18N2O4S ∙ C13H20N2O2 ∙ H2O | Molecular Wt.
588.72 | |
Each 2 mL syringe contains the equivalent of 1,200,000 units of penicillin G as follows: penicillin G benzathine equivalent to 900,000 units of penicillin G and penicillin G procaine equivalent to 300,000 units of penicillin G in a stabilized aqueous suspension with sodium citrate buffer; and as w/v, approximately 0.5% lecithin, 0.55% carboxymethylcellulose, 0.55% povidone, 0.1% methylparaben, and 0.01% propylparaben.
Bicillin C-R 900/300 injectable suspension is viscous and opaque. Read CONTRAINDICATIONS, WARNINGS, PRECAUTIONS, and DOSAGE AND ADMINISTRATION sections prior to use.