BESPONSA™ Clinical Studies
(inotuzumab ozogamicin)
14. CLINICAL STUDIES
Relapsed or Refractory ALL
INO-VATE ALL Study – Adult Patients
The safety and efficacy of BESPONSA were evaluated in INO-VATE ALL (NCT01564784) a randomized (1:1), open‑label, international, multicenter study in patients with relapsed or refractory ALL. Patients were stratified at randomization based on duration of first remission (< 12 months or ≥ 12 months, salvage treatment (Salvage 1 or 2) and patient age at randomization (< 55 or ≥ 55 years). Eligible patients were ≥ 18 years of age with Philadelphia chromosome-negative or Philadelphia chromosome-positive relapsed or refractory B-cell precursor ALL. All patients were required to have ≥ 5% bone marrow blasts and to have received 1 or 2 previous induction chemotherapy regimens for ALL. Patients with Philadelphia chromosome-positive B-cell precursor ALL were required to have disease that failed treatment with at least 1 tyrosine kinase inhibitor and standard chemotherapy. Table 1 shows the dosing regimen used to treat patients.
Among all 326 patients who were randomized to receive BESPONSA (N=164) or Investigator's choice of chemotherapy (N=162), 215 patients (66%) had received 1 prior treatment regimen for ALL and 108 patients (33%) had received 2 prior treatment regimens for ALL. The median age was 47 years (range: 18–79 years), 276 patients (85%) had Philadelphia chromosome-negative ALL, 206 patients (63%) had a duration of first remission < 12 months, and 55 patients (17%) had undergone a HSCT prior to receiving BESPONSA or Investigator's choice of chemotherapy. The two treatment groups were generally balanced with respect to the baseline demographics and disease characteristics.
All evaluable patients had B-cell precursor ALL that expressed CD22, with ≥ 90% of evaluable patients exhibiting ≥ 70% leukemic blast CD22 positivity prior to treatment, as assessed by flow cytometry performed at a central laboratory.
The efficacy of BESPONSA was established on the basis of CR, the duration of CR, and proportion of MRD-negative CR (< 1 × 10-4 of bone marrow nucleated cells by flow cytometry) in the first 218 patients randomized. CR, duration of remission (DoR), and MRD results in the initial 218 randomized patients were consistent with those seen in all 326 randomized patients.
Among the initial 218 randomized patients, 64/88 (73%) and 21/88 (24%) of responding patients per EAC achieved CR/CRi in Cycles 1 and 2, respectively, in the BESPONSA arm, and 29/32 (91%) and 1/32 (3%) of responding patients per EAC achieved a CR/CRi in Cycles 1 and 2, respectively, in the Investigator's choice of chemotherapy arm.
Table 11 shows the efficacy results from this study.
| CR* | CRi† | CR/CRi*,† | ||||
|---|---|---|---|---|---|---|
| BESPONSA (N=109) | HIDAC, FLAG, or MXN/Ara-C (N=109) | BESPONSA (N=109) | HIDAC, FLAG or MXN/Ara-C (N=109) | BESPONSA (N=109) | HIDAC, FLAG, or MXN/Ara-C (N=109) | |
| Abbreviations: CI=confidence interval; CR=complete remission; CRi=complete remission with incomplete hematologic recovery; DoR=duration of remission; EAC=Endpoint Adjudication Committee; FLAG=fludarabine + cytarabine + granulocyte colony-stimulating factor; HIDAC=high-dose cytarabine; HR=hazard ratio; MRD=minimal residual disease; MXN/AraC=mitoxantrone + cytarabine; N/n=number of patients; OS=overall survival; PFS=progression-free survival. | ||||||
| ||||||
Responding (CR/CRi) patients | ||||||
n (%) | 39 (35.8) | 19 (17.4) | 49 (45.0) | 13 (11.9) | 88 (80.7) | 32 (29.4) |
p-value‡ | < 0.0001 | |||||
DoR§ | ||||||
n | 39 | 18 | 45 | 14 | 84 | 32 |
Median, months | 8.0 | 4.9 | 4.6 | 2.9 | 5.4 | 3.5 |
MRD-negativity¶ | ||||||
n | 35 | 6 | 34 | 3 | 69 | 9 |
Rate# (%) | 35/39 (89.7) | 6/19 (31.6) | 34/49 (69.4) | 3/13 (23.1) | 69/88 (78.4) | 9/32 (28.1) |
Among the initial 218 patients, as per EAC assessment, 32/109 patients (29%) in the BESPONSA arm achieved complete remission with partial hematologic recovery (CRh; defined as < 5% blasts in the bone marrow, ANC > 0.5 × 109/L, and platelet counts > 50 × 109/L but not meeting full recovery of peripheral blood counts) versus 6/109 patients (6%) in the Investigator's choice of chemotherapy arm, and 71/109 patients (65%) in the BESPONSA arm achieved CR/CRh versus 25/109 patients (23%) in the Investigator's choice of chemotherapy arm.
Overall, 79/164 patients (48%) in the BESPONSA arm and 35/162 patients (22%) in the Investigator's choice of chemotherapy arm had a follow-up HSCT.
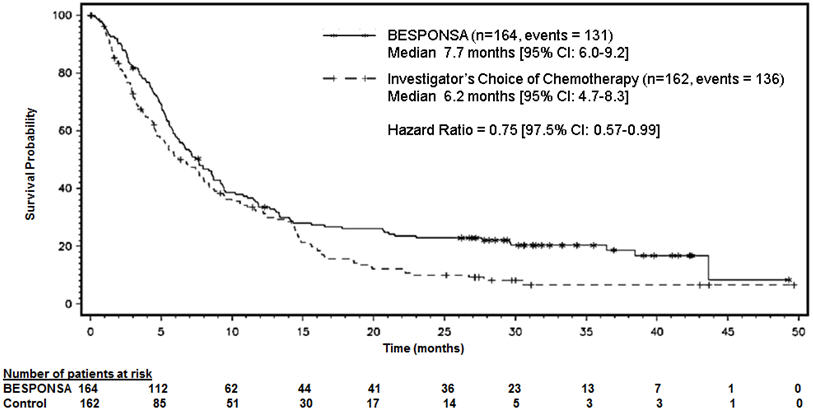
Figure 1 shows the analysis of overall survival (OS). The analysis of OS did not meet the pre-specified boundary for statistical significance.
Figure 1. Kaplan-Meier Curve for Overall Survival (Intent-to-Treat Population)
WI203581 (ITCC-059) – Pediatric Patients
BESPONSA was evaluated in a multicenter, single-arm, open-label study in 53 pediatric patients ≥ 1 and < 18 years of age with relapsed or refractory CD22-positive B-cell precursor ALL.
In 53 patients, there were two dose levels: an initial dose of 1.4 mg/m2/cycle (approximately 0.78 times the recommended initial dosage) in 12 patients and 1.8 mg/m2/cycle in 41 patients (premedications included methylprednisolone 1 mg/kg with a maximum of 50 mg, an antipyretic, and an antihistamine). Table 1 shows the dosing regimen used to treat patients. Patients received a median of 2 cycles of therapy (range: 1 to 4 cycles). The median age was 9 years (range: 1 to 17 years), and 55% of patients had second or greater relapsed B-cell precursor ALL.
Efficacy was established on the basis of the Complete Remission (CR) Rate [CR was defined as < 5% blasts in the bone marrow and the absence of peripheral blood leukemic blasts, full recovery of peripheral blood counts (platelets ≥ 100 × 109/L and ANC ≥ 1 × 109/L) and resolution of any extramedullary disease], duration of CR, and proportion of patients with MRD negative CR [MRD was defined by leukemic cells comprising < 1 × 10-4 (< 0.01%) of bone marrow nucleated cells by flow cytometry or by PCR]. In all patients, 22/53 (42%, 95% CI 28.1-55.9%) patients achieved CR, and the median duration of CR (DOCR) was 8.2 months (95% CI: 2.6-NE). The minimal residual disease (MRD) negativity rate in patients with CR was 21/22 [95.5% (95% CI: 77.2-99.9)] based on flow cytometry, and 19/22 [86.4% (95% CI: 65.1-97.1)] based on RQ-PCR.
Find BESPONSA™ medical information:
Find BESPONSA™ medical information:
BESPONSA™ Quick Finder
Health Professional Information
Clinical Studies
14. CLINICAL STUDIES
Relapsed or Refractory ALL
INO-VATE ALL Study – Adult Patients
The safety and efficacy of BESPONSA were evaluated in INO-VATE ALL (NCT01564784) a randomized (1:1), open‑label, international, multicenter study in patients with relapsed or refractory ALL. Patients were stratified at randomization based on duration of first remission (< 12 months or ≥ 12 months, salvage treatment (Salvage 1 or 2) and patient age at randomization (< 55 or ≥ 55 years). Eligible patients were ≥ 18 years of age with Philadelphia chromosome-negative or Philadelphia chromosome-positive relapsed or refractory B-cell precursor ALL. All patients were required to have ≥ 5% bone marrow blasts and to have received 1 or 2 previous induction chemotherapy regimens for ALL. Patients with Philadelphia chromosome-positive B-cell precursor ALL were required to have disease that failed treatment with at least 1 tyrosine kinase inhibitor and standard chemotherapy. Table 1 shows the dosing regimen used to treat patients.
Among all 326 patients who were randomized to receive BESPONSA (N=164) or Investigator's choice of chemotherapy (N=162), 215 patients (66%) had received 1 prior treatment regimen for ALL and 108 patients (33%) had received 2 prior treatment regimens for ALL. The median age was 47 years (range: 18–79 years), 276 patients (85%) had Philadelphia chromosome-negative ALL, 206 patients (63%) had a duration of first remission < 12 months, and 55 patients (17%) had undergone a HSCT prior to receiving BESPONSA or Investigator's choice of chemotherapy. The two treatment groups were generally balanced with respect to the baseline demographics and disease characteristics.
All evaluable patients had B-cell precursor ALL that expressed CD22, with ≥ 90% of evaluable patients exhibiting ≥ 70% leukemic blast CD22 positivity prior to treatment, as assessed by flow cytometry performed at a central laboratory.
The efficacy of BESPONSA was established on the basis of CR, the duration of CR, and proportion of MRD-negative CR (< 1 × 10-4 of bone marrow nucleated cells by flow cytometry) in the first 218 patients randomized. CR, duration of remission (DoR), and MRD results in the initial 218 randomized patients were consistent with those seen in all 326 randomized patients.
Among the initial 218 randomized patients, 64/88 (73%) and 21/88 (24%) of responding patients per EAC achieved CR/CRi in Cycles 1 and 2, respectively, in the BESPONSA arm, and 29/32 (91%) and 1/32 (3%) of responding patients per EAC achieved a CR/CRi in Cycles 1 and 2, respectively, in the Investigator's choice of chemotherapy arm.
Table 11 shows the efficacy results from this study.
| CR* | CRi† | CR/CRi*,† | ||||
|---|---|---|---|---|---|---|
| BESPONSA (N=109) | HIDAC, FLAG, or MXN/Ara-C (N=109) | BESPONSA (N=109) | HIDAC, FLAG or MXN/Ara-C (N=109) | BESPONSA (N=109) | HIDAC, FLAG, or MXN/Ara-C (N=109) | |
| Abbreviations: CI=confidence interval; CR=complete remission; CRi=complete remission with incomplete hematologic recovery; DoR=duration of remission; EAC=Endpoint Adjudication Committee; FLAG=fludarabine + cytarabine + granulocyte colony-stimulating factor; HIDAC=high-dose cytarabine; HR=hazard ratio; MRD=minimal residual disease; MXN/AraC=mitoxantrone + cytarabine; N/n=number of patients; OS=overall survival; PFS=progression-free survival. | ||||||
| ||||||
Responding (CR/CRi) patients | ||||||
n (%) | 39 (35.8) | 19 (17.4) | 49 (45.0) | 13 (11.9) | 88 (80.7) | 32 (29.4) |
p-value‡ | < 0.0001 | |||||
DoR§ | ||||||
n | 39 | 18 | 45 | 14 | 84 | 32 |
Median, months | 8.0 | 4.9 | 4.6 | 2.9 | 5.4 | 3.5 |
MRD-negativity¶ | ||||||
n | 35 | 6 | 34 | 3 | 69 | 9 |
Rate# (%) | 35/39 (89.7) | 6/19 (31.6) | 34/49 (69.4) | 3/13 (23.1) | 69/88 (78.4) | 9/32 (28.1) |
Among the initial 218 patients, as per EAC assessment, 32/109 patients (29%) in the BESPONSA arm achieved complete remission with partial hematologic recovery (CRh; defined as < 5% blasts in the bone marrow, ANC > 0.5 × 109/L, and platelet counts > 50 × 109/L but not meeting full recovery of peripheral blood counts) versus 6/109 patients (6%) in the Investigator's choice of chemotherapy arm, and 71/109 patients (65%) in the BESPONSA arm achieved CR/CRh versus 25/109 patients (23%) in the Investigator's choice of chemotherapy arm.
Overall, 79/164 patients (48%) in the BESPONSA arm and 35/162 patients (22%) in the Investigator's choice of chemotherapy arm had a follow-up HSCT.
Figure 1 shows the analysis of overall survival (OS). The analysis of OS did not meet the pre-specified boundary for statistical significance.
Figure 1. Kaplan-Meier Curve for Overall Survival (Intent-to-Treat Population)
WI203581 (ITCC-059) – Pediatric Patients
BESPONSA was evaluated in a multicenter, single-arm, open-label study in 53 pediatric patients ≥ 1 and < 18 years of age with relapsed or refractory CD22-positive B-cell precursor ALL.
In 53 patients, there were two dose levels: an initial dose of 1.4 mg/m2/cycle (approximately 0.78 times the recommended initial dosage) in 12 patients and 1.8 mg/m2/cycle in 41 patients (premedications included methylprednisolone 1 mg/kg with a maximum of 50 mg, an antipyretic, and an antihistamine). Table 1 shows the dosing regimen used to treat patients. Patients received a median of 2 cycles of therapy (range: 1 to 4 cycles). The median age was 9 years (range: 1 to 17 years), and 55% of patients had second or greater relapsed B-cell precursor ALL.
Efficacy was established on the basis of the Complete Remission (CR) Rate [CR was defined as < 5% blasts in the bone marrow and the absence of peripheral blood leukemic blasts, full recovery of peripheral blood counts (platelets ≥ 100 × 109/L and ANC ≥ 1 × 109/L) and resolution of any extramedullary disease], duration of CR, and proportion of patients with MRD negative CR [MRD was defined by leukemic cells comprising < 1 × 10-4 (< 0.01%) of bone marrow nucleated cells by flow cytometry or by PCR]. In all patients, 22/53 (42%, 95% CI 28.1-55.9%) patients achieved CR, and the median duration of CR (DOCR) was 8.2 months (95% CI: 2.6-NE). The minimal residual disease (MRD) negativity rate in patients with CR was 21/22 [95.5% (95% CI: 77.2-99.9)] based on flow cytometry, and 19/22 [86.4% (95% CI: 65.1-97.1)] based on RQ-PCR.
Health Professional Information
{{section_name_patient}}
{{section_body_html_patient}}
Resources
Didn’t find what you were looking for? Contact us.
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine.
*Speak with a Pfizer Medical Information Professional regarding your medical inquiry. Available 9AM-5Pm ET Monday to Friday; excluding holidays.
Submit a medical question for Pfizer prescription products.
Report Adverse Event
To report an adverse event related to the Pfizer-BioNTech COVID-19 Vaccine, and you are not part of a clinical trial* for this product, click the link below to submit your information:
Pfizer Safety Reporting Site*If you are involved in a clinical trial for this product, adverse events should be reported to your coordinating study site.
If you cannot use the above website, or would like to report an adverse event related to a different Pfizer product, please call Pfizer Safety at (800) 438-1985.
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or call (800) 822-7967.