atropine sulfate injection, USP
()
Find atropine sulfate injection, USP medical information:
Find atropine sulfate injection, USP medical information:
atropine sulfate injection, USP Quick Finder
Highlights
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ATROPINE SULFATE INJECTION safely and effectively. See full prescribing information for ATROPINE SULFATE INJECTION. ATROPINE SULFATE INJECTION, for intravenous use Initial U.S. Approval: 1960 INDICATIONS AND USAGEAtropine is a muscarinic antagonist indicated for temporary blockade of severe or life threatening muscarinic effects. (1) DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHSCONTRAINDICATIONSNone. (4) WARNINGS AND PRECAUTIONSADVERSE REACTIONSMost adverse reactions are directly related to atropine's antimuscarinic action. Dryness of the mouth, blurred vision, photophobia and tachycardia commonly occur with chronic administration of therapeutic doses. (6) To report SUSPECTED ADVERSE REACTIONS, contact Hospira, Inc. at 1-800-441-4100, or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. DRUG INTERACTIONSMexiletine: Decreases rate of mexiletine absorption. (7.1) Revised: 6/2020 |
Indications and Usage
1 INDICATIONS AND USAGE
Atropine Sulfate Injection, USP, is indicated for temporary blockade of severe or life threatening muscarinic effects, e.g., as an antisialagogue, an antivagal agent, an antidote for organophosphorus or muscarinic mushroom poisoning, and to treat bradyasystolic cardiac arrest.
Dosage and Administration
2 DOSAGE AND ADMINISTRATION
2.1 General Administration
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Do not administer unless solution is clear and seal is intact. Each syringe is intended for single dose only. Discard unused portion.
For intravenous administration.
Titrate based on heart rate, PR interval, blood pressure and symptoms.
2.2 Adult Dosage
Use | Dose (adults) | Repeat |
Antisialagogue or other antivagal | 0.5 to 1 mg | 1-2 hours |
Organophosphorus or muscarinic mushroom poisoning | 2 to 3 mg | 20-30 minutes |
Bradyasystolic cardiac arrest | 1 mg | 3-5 minutes; 3 mg maximum total dose |
2.3 Pediatric Dosage
Dosing in pediatric populations has not been well studied. Usual initial dose is 0.01 to 0.03 mg/kg.
2.4 Dosing in Patients with Coronary Artery Disease
Limit the total dose of atropine sulfate to 0.03 mg/kg to 0.04 mg/kg [see Warnings and Precautions (5.1)].
Dosage Forms and Strengths
Contraindications
Warnings and Precautions
5 WARNINGS AND PRECAUTIONS
5.1 Tachycardia
When the recurrent use of atropine is essential in patients with coronary artery disease, the total dose should be restricted to 2 to 3 mg (maximum 0.03 to 0.04 mg/kg) to avoid the detrimental effects of atropine-induced tachycardia on myocardial oxygen demand.
5.3 Pyloric Obstruction
Atropine may convert partial organic pyloric stenosis into complete obstruction.
Adverse Reactions
6 ADVERSE REACTIONS
The following adverse reactions have been identified during post-approval use of atropine sulfate. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Most of the side effects of atropine are directly related to its antimuscarinic action. Dryness of the mouth, blurred vision, photophobia and tachycardia commonly occur. Anhidrosis can produce heat intolerance. Constipation and difficulty in micturition may occur in elderly patients. Occasional hypersensitivity reactions have been observed, especially skin rashes which in some instances progressed to exfoliation.
Drug Interactions
Use in Specific Populations
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Animal reproduction studies have not been conducted with atropine. It also is not known whether atropine can cause fetal harm when given to a pregnant woman or can affect reproduction capacity.
8.3 Nursing Mothers
Trace amounts of atropine was found in breast milk. The clinical impact of this is not known.
8.5 Geriatric Use
An evaluation of current literature revealed no clinical experience identifying differences in response between elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Overdosage
10 OVERDOSAGE
Excessive dosing may cause palpitation, dilated pupils, difficulty in swallowing, hot dry skin, thirst, dizziness, restlessness, tremor, fatigue and ataxia. Toxic doses lead to restlessness and excitement, hallucinations, delirium and coma. Depression and circulatory collapse occur only with severe intoxication. In such cases, blood pressure declines and death due to respiratory failure may ensue following paralysis and coma.
The fatal adult dose of atropine is not known. In pediatric populations, 10 mg or less may be fatal.
In the event of toxic overdosage, a short acting barbiturate or diazepam may be given as needed to control marked excitement and convulsions. Large doses for sedation should be avoided because central depressant action may coincide with the depression occurring late in atropine poisoning. Central stimulants are not recommended.
Physostigmine, given as an atropine antidote by slow intravenous injection of 1 to 4 mg (0.5 to 1 mg in pediatric populations), rapidly abolishes delirium and coma caused by large doses of atropine. Since physostigmine is rapidly destroyed, the patient may again lapse into coma after one to two hours, and repeated doses may be required.
Artificial respiration with oxygen may be necessary. Ice bags and alcohol sponges help to reduce fever, especially in pediatric populations.
Atropine is not removed by dialysis.
Description
11 DESCRIPTION
Atropine Sulfate Injection, USP is a sterile, nonpyrogenic isotonic solution of atropine sulfate monohydrate in water for injection with sodium chloride sufficient to render the solution isotonic. It is administered parenterally by intravenous injection.
Each milliliter (mL) contains 0.1 mg (adult strength) or 0.05 mg (pediatric strength) of atropine sulfate monohydrate equivalent to 0.083 mg (adult strength) or 0.042 mg (pediatric strength) of atropine, and sodium chloride, 9 mg. May contain sodium hydroxide and/or sulfuric acid for pH adjustment 0.308 mOsmol/mL (calc.). pH 3.0 to 6.5.
Sodium chloride added to render the solution isotonic for injection of the active ingredient is present in amounts insufficient to affect serum electrolyte balance of sodium (Na+) and chloride (Cl-) ions.
The solution contains no bacteriostat, antimicrobial agent or added buffer (except for pH adjustment) and is intended for use only as a single-dose injection. When smaller doses are required the unused portion should be discarded.
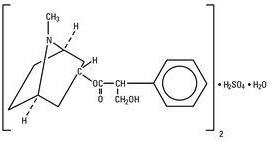
Atropine Sulfate, USP is chemically designated 1α H, 5α H-Tropan-3-α-ol (±)-tropate (ester), sulfate (2:1) (salt) monohydrate, (C17H23NO3)2 H2SO4 H2O, colorless crystals or white crystalline powder very soluble in water. It has the following structural formula:
Atropine, a naturally occurring belladonna alkaloid, is a racemic mixture of equal parts of d- and 1-hyocyamine, whose activity is due almost entirely to the levo isomer of the drug.
Sodium Chloride, USP is chemically designated NaCl, a white crystalline powder freely soluble in water.
The Ansyr™ syringe is molded from a specially formulated polypropylene. Water permeates from inside the container at an extremely slow rate which will have an insignificant effect on solution concentration over the expected shelf life. Solutions in contact with the plastic container may leach out certain chemical components from the plastic in very small amounts; however, biological testing was supportive of the safety of the syringe material.
Clinical Pharmacology
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Atropine is an antimuscarinic agent since it antagonizes the muscarine-like actions of acetylcholine and other choline esters.
Atropine inhibits the muscarinic actions of acetylcholine on structures innervated by postganglionic cholinergic nerves, and on smooth muscles which respond to endogenous acetylcholine but are not so innervated. As with other antimuscarinic agents, the major action of atropine is a competitive or surmountable antagonism which can be overcome by increasing the concentration of acetylcholine at receptor sites of the effector organ (e.g., by using anticholinesterase agents which inhibit the enzymatic destruction of acetylcholine). The receptors antagonized by atropine are the peripheral structures that are stimulated or inhibited by muscarine (i.e., exocrine glands and smooth and cardiac muscle). Responses to postganglionic cholinergic nerve stimulation also may be inhibited by atropine but this occurs less readily than with responses to injected (exogenous) choline esters.
12.2 Pharmacodynamics
Atropine-induced parasympathetic inhibition may be preceded by a transient phase of stimulation, especially on the heart where small doses first slow the rate before characteristic tachycardia develops due to paralysis of vagal control. Atropine exerts a more potent and prolonged effect on heart, intestine and bronchial muscle than scopolamine, but its action on the iris, ciliary body and certain secretory glands is weaker than that of scopolamine. Unlike the latter, atropine in clinical doses does not depress the central nervous system but may stimulate the medulla and higher cerebral centers. Although mild vagal excitation occurs, the increased respiratory rate and (sometimes) increased depth of respiration produced by atropine are more probably the result of bronchiolar dilatation. Accordingly, atropine is an unreliable respiratory stimulant and large or repeated doses may depress respiration.
Adequate doses of atropine abolish various types of reflex vagal cardiac slowing or asystole. The drug also prevents or abolishes bradycardia or asystole produced by injection of choline esters, anticholinesterase agents or other parasympathomimetic drugs, and cardiac arrest produced by stimulation of the vagus. Atropine also may lessen the degree of partial heart block when vagal activity is an etiologic factor. In some patients with complete heart block, the idioventricular rate may be accelerated by atropine; in others, the rate is stabilized. Occasionally a large dose may cause atrioventricular (A-V) block and nodal rhythm.
Atropine Sulfate Injection, USP in clinical doses counteracts the peripheral dilatation and abrupt decrease in blood pressure produced by choline esters. However, when given by itself, atropine does not exert a striking or uniform effect on blood vessels or blood pressure. Systemic doses slightly raise systolic and lower diastolic pressures and can produce significant postural hypotension. Such doses also slightly increase cardiac output and decrease central venous pressure. Occasionally, therapeutic doses dilate cutaneous blood vessels, particularly in the "blush" area (atropine flush), and may cause atropine "fever" due to suppression of sweat gland activity in infants and small children.
The effects of intravenous atropine on heart rate (maximum heart rate) and saliva flow (minimum flow) after intravenous administration (rapid, constant infusion over 3 min.) are delayed by 7 to 8 minutes after drug administration and both effects are non-linearly related to the amount of drug in the peripheral compartment. Changes in plasma atropine levels following intramuscular administration (0.5 to 4 mg doses) and heart rate are closely overlapped but the time course of the changes in atropine levels and behavioral impairment indicates that pharmacokinetics is not the primary rate-limiting mechanism for the central nervous system effect of atropine.
12.3 Pharmacokinetics
Atropine disappears rapidly from the blood following injection and is distributed throughout the body. Exercise, both prior to and immediately following intramuscular administration of atropine, significantly increases the absorption of atropine due to increased perfusion in the muscle and significantly decreases the clearance of atropine. The pharmacokinetics of atropine is nonlinear after intravenous administration of 0.5 to 4 mg. Atropine's plasma protein binding is about 44% and saturable in the 2-20 μg/mL concentration range. Atropine readily crosses the placental barrier and enters the fetal circulation, but is not found in amniotic fluid. Much of the drug is destroyed by enzymatic hydrolysis, particularly in the liver; from 13 to 50% is excreted unchanged in the urine. Traces are found in various secretions, including milk. The major metabolites of atropine are noratropine, atropin-n-oxide, tropine, and tropic acid. The metabolism of atropine is inhibited by organophosphate pesticides.
Specific Populations
The elimination half-life of atropine is more than doubled in children under two years and the elderly (>65 years old) compared to other age groups. There is no gender effect on the pharmacokinetics and pharmacodynamics (heart rate changes) of atropine.
Nonclinical Toxicology
How Supplied/Storage and Handling
16 HOW SUPPLIED/STORAGE AND HANDLING
Atropine Sulfate Injection, USP is supplied in single-dose syringes as follows:
Syringes | Concentration (mg/mL) | Fill Volume | Total Atropine Content | NDC# |
Ansyr™ Plastic Syringe | 0.1 mg/mL | 10 mL | 1 mg | 0409-1630-10 |
Ansyr™ Plastic Syringe | 0.05 mg/mL | 5 mL | 0.25 mg | 0409-9630-05 |
LifeShield™ Abboject™ Glass Syringe | 0.1 mg/mL | 5 mL | 0.5 mg | 0409-4910-34 |
LifeShield™ Abboject™ Glass Syringe | 0.1 mg/mL | 10 mL | 1 mg | 0409-4911-34 |
Store at 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C and 30°C (59°F and 86°F). [See USP Controlled Room Temperature.]
Distributed by Hospira, Inc., Lake Forest, IL 60045 USA 
Abboject™ is a trademark of the Abbott group of companies.
LifeShield™ is the trademark of ICU Medical, Inc. and is used under license.
LAB-1041-4.0
Resources
Didn’t find what you were looking for?
Contact us.
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine.
*Speak with a Pfizer Medical Information Professional regarding your medical inquiry. Available 9AM-5Pm ET Monday to Friday; excluding holidays.
Submit a medical question for Pfizer prescription products.
Report Adverse Event
To report an adverse event related to a Pfizer product and you are not part of a clinical trial* for this product, click the link below to submit your information: Pfizer Safety Reporting Site
*If you are involved in a clinical trial for either product, adverse events should be reported to your coordinating study site.
If you cannot use the above website to report an adverse event related to a Pfizer product, please call Pfizer Medical Information at (800) 438-1985.
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or by calling (800)-332-1088.