docetaxel injection, USP 20 mg/ml VIAL Description
11 DESCRIPTION
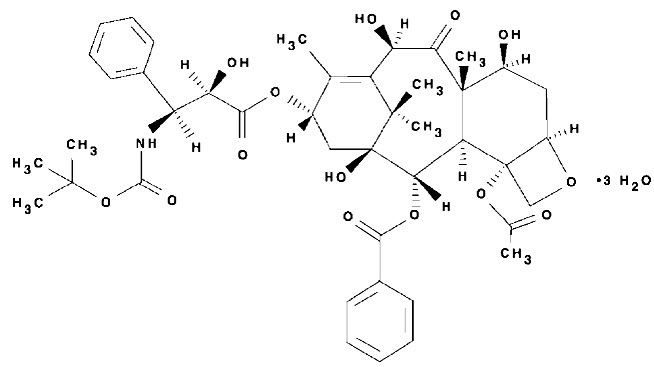
Docetaxel is an antineoplastic agent belonging to the taxoid family. It is prepared by semisynthesis beginning with a precursor extracted from the renewable needle biomass of yew plants. The chemical name for docetaxel is (2R,3S)-N-carboxy-3-phenylisoserine,N-tert-butyl ester, 13-ester with 5β-20-epoxy-1,2α,4,7β,10β,13α-hexahydroxytax-11-en-9-one 4-acetate 2-benzoate, trihydrate. Docetaxel (trihydrate) has the following structural formula:
Docetaxel (trihydrate) is a white to almost-white powder with an empirical formula of C43H53NO14 ∙ 3H2O, and a molecular weight of 861.9. It is highly lipophilic and practically insoluble in water.
Docetaxel Injection, USP is a sterile, non-pyrogenic, pale-yellow to brownish yellow solution at 20 mg/mL concentration.
Each mL contains 20 mg docetaxel (anhydrous), 520 mg polysorbate 80 NF, 7 mg citric acid monohydrate USP, and 395 mg dehydrated alcohol USP.
Docetaxel Injection is available in single-dose vials containing 20 mg (1 mL) or multiple-dose vials containing 80 mg (4 mL) or 160 mg (8 mL) docetaxel (anhydrous).
Docetaxel Injection requires NO prior dilution with a diluent and is ready to add to the infusion solution.
Find docetaxel injection, USP 20 mg/ml VIAL medical information:
Find docetaxel injection, USP 20 mg/ml VIAL medical information:
docetaxel injection, USP 20 mg/ml VIAL Quick Finder
Health Professional Information
Description
11 DESCRIPTION
Docetaxel is an antineoplastic agent belonging to the taxoid family. It is prepared by semisynthesis beginning with a precursor extracted from the renewable needle biomass of yew plants. The chemical name for docetaxel is (2R,3S)-N-carboxy-3-phenylisoserine,N-tert-butyl ester, 13-ester with 5β-20-epoxy-1,2α,4,7β,10β,13α-hexahydroxytax-11-en-9-one 4-acetate 2-benzoate, trihydrate. Docetaxel (trihydrate) has the following structural formula:
Docetaxel (trihydrate) is a white to almost-white powder with an empirical formula of C43H53NO14 ∙ 3H2O, and a molecular weight of 861.9. It is highly lipophilic and practically insoluble in water.
Docetaxel Injection, USP is a sterile, non-pyrogenic, pale-yellow to brownish yellow solution at 20 mg/mL concentration.
Each mL contains 20 mg docetaxel (anhydrous), 520 mg polysorbate 80 NF, 7 mg citric acid monohydrate USP, and 395 mg dehydrated alcohol USP.
Docetaxel Injection is available in single-dose vials containing 20 mg (1 mL) or multiple-dose vials containing 80 mg (4 mL) or 160 mg (8 mL) docetaxel (anhydrous).
Docetaxel Injection requires NO prior dilution with a diluent and is ready to add to the infusion solution.
Health Professional Information
{{section_name_patient}}
{{section_body_html_patient}}
Resources
Didn’t find what you were looking for? Contact us.
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine.
*Speak with a Pfizer Medical Information Professional regarding your medical inquiry. Available 9AM-5PM ET Monday to Friday; excluding holidays.
Submit a medical question for Pfizer prescription products.
Report Adverse Event
Pfizer Safety
To report an adverse event related to the Pfizer-BioNTech COVID-19 Vaccine, and you are not part of a clinical trial* for this product, click the link below to submit your information:
Pfizer Safety Reporting Site*If you are involved in a clinical trial for this product, adverse events should be reported to your coordinating study site.
If you cannot use the above website, or would like to report an adverse event related to a different Pfizer product, please call Pfizer Safety at (800) 438-1985.
FDA Medwatch
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or call (800) 822-7967.