dobutamine in 5% dextrose injection, USP Description
DESCRIPTION
Dobutamine in 5% Dextrose Injection, USP is a sterile, nonpyrogenic, prediluted solution of dobutamine hydrochloride and dextrose in water for injection. It is administered by intravenous infusion.
Each 100 mL contains dobutamine hydrochloride equivalent to 100 mg, 200 mg, or 400 mg of dobutamine; dextrose (derived from corn), hydrous 5 g in water for injection, with sodium metabisulfite 25 mg and edetate disodium, dihydrate 10 mg added as stabilizers; osmolar concentration, respectively, 263, 270, or 284 mOsmol/liter (calc.). The pH is 3.0 (2.5 to 5.5). May contain hydrochloric acid and/or sodium hydroxide for pH adjustment. Dobutamine in 5% Dextrose Injection, USP is oxygen sensitive.
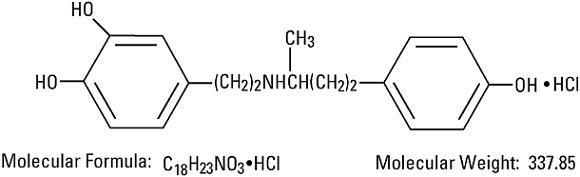
Dobutamine Hydrochloride, USP is chemically designated (±)-4-[2-[[3-(p-hydroxyphenyl)-1-methylpropyl]amino]ethyl]-pyrocatechol hydrochloride. It is a synthetic catecholamine.
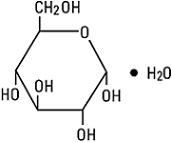
Dextrose, USP is chemically designated D-glucose monohydrate (C6H12O6 • H2O), a hexose sugar freely soluble in water. It has the following structural formula:
Water for Injection, USP is chemically designated H2O.
The flexible plastic container is fabricated from a specially formulated CR3 plastic material. Water can permeate from inside the container into the overwrap but not in amounts sufficient to affect the solution significantly. Solutions in contact with the plastic container may leach out certain chemical components from the plastic in very small amounts; however, biological testing was supportive of the safety of the plastic container materials. Exposure to temperatures above 25°C/77°F during transport and storage will lead to minor losses in moisture content. Higher temperatures lead to greater losses. It is unlikely that these minor losses will lead to clinically significant changes within the expiration period.
Find dobutamine in 5% dextrose injection, USP medical information:
Find dobutamine in 5% dextrose injection, USP medical information:
dobutamine in 5% dextrose injection, USP Quick Finder
Health Professional Information
Description
DESCRIPTION
Dobutamine in 5% Dextrose Injection, USP is a sterile, nonpyrogenic, prediluted solution of dobutamine hydrochloride and dextrose in water for injection. It is administered by intravenous infusion.
Each 100 mL contains dobutamine hydrochloride equivalent to 100 mg, 200 mg, or 400 mg of dobutamine; dextrose (derived from corn), hydrous 5 g in water for injection, with sodium metabisulfite 25 mg and edetate disodium, dihydrate 10 mg added as stabilizers; osmolar concentration, respectively, 263, 270, or 284 mOsmol/liter (calc.). The pH is 3.0 (2.5 to 5.5). May contain hydrochloric acid and/or sodium hydroxide for pH adjustment. Dobutamine in 5% Dextrose Injection, USP is oxygen sensitive.
Dobutamine Hydrochloride, USP is chemically designated (±)-4-[2-[[3-(p-hydroxyphenyl)-1-methylpropyl]amino]ethyl]-pyrocatechol hydrochloride. It is a synthetic catecholamine.
Dextrose, USP is chemically designated D-glucose monohydrate (C6H12O6 • H2O), a hexose sugar freely soluble in water. It has the following structural formula:
Water for Injection, USP is chemically designated H2O.
The flexible plastic container is fabricated from a specially formulated CR3 plastic material. Water can permeate from inside the container into the overwrap but not in amounts sufficient to affect the solution significantly. Solutions in contact with the plastic container may leach out certain chemical components from the plastic in very small amounts; however, biological testing was supportive of the safety of the plastic container materials. Exposure to temperatures above 25°C/77°F during transport and storage will lead to minor losses in moisture content. Higher temperatures lead to greater losses. It is unlikely that these minor losses will lead to clinically significant changes within the expiration period.
Resources
Didn’t find what you were looking for? Contact us.
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine.
*Speak with a Pfizer Medical Information Professional regarding your medical inquiry. Available 9AM-5PM ET Monday to Friday; excluding holidays.
Submit a medical question for Pfizer prescription products.
Report Adverse Event
Pfizer Safety
To report an adverse event related to the Pfizer-BioNTech COVID-19 Vaccine, and you are not part of a clinical trial* for this product, click the link below to submit your information:
Pfizer Safety Reporting Site*If you are involved in a clinical trial for this product, adverse events should be reported to your coordinating study site.
If you cannot use the above website, or would like to report an adverse event related to a different Pfizer product, please call Pfizer Safety at (800) 438-1985.
FDA Medwatch
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or call (800) 822-7967.