bleomycin for Injection, USP Description
DESCRIPTION
Bleomycin for Injection, USP is a mixture of cytotoxic glycopeptide antibiotics isolated from a strain of Streptomyces verticillus. It is freely soluble in water.
It is available as a lyophilized powder for intramuscular, intravenous or subcutaneous injection. Each vial contains sterile bleomycin sulphate equivalent to 15 units or 30 units of bleomycin.
Sulfuric acid or Sodium hydroxide used, if necessary to adjust the pH.
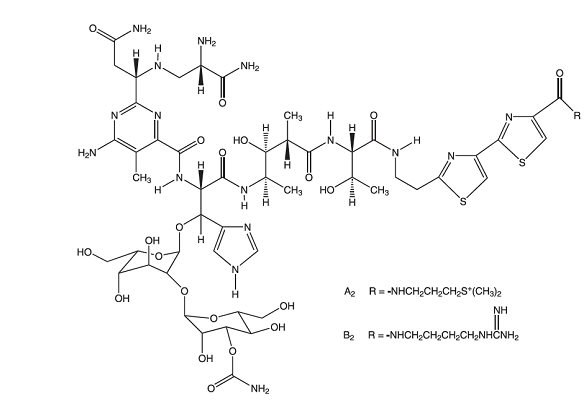
Bleomycins are a group of related basic glycopeptides which differ in the terminal amine substituent of the common structural unit, bleomycin acid. The main components of Bleomycin for Injection are bleomycins A2 and B2. Chemically, bleomycin A2 is N1-[3-(dimethylsulfonio)propyl]-bleomycinamide and bleomycin B2 is N1-[4-(aminoiminomethyl)amino]butyl]-bleomycinamide.
The molecular formula of bleomycin A2 is C55H84N17O21S3 and a calculated molecular weight of 1414. The molecular formula of bleomycin B2 is C55H84N20O21S2 and a calculated molecular weight of 1425. The structural formula of bleomycins A2 and B2 are shown below.
Note: A unit of bleomycin is equal to the formerly used milligram activity. The term milligram activity is a misnomer and was changed to units to be more precise.
Find bleomycin for Injection, USP medical information:
Find bleomycin for Injection, USP medical information:
bleomycin for Injection, USP Quick Finder
Health Professional Information
Description
DESCRIPTION
Bleomycin for Injection, USP is a mixture of cytotoxic glycopeptide antibiotics isolated from a strain of Streptomyces verticillus. It is freely soluble in water.
It is available as a lyophilized powder for intramuscular, intravenous or subcutaneous injection. Each vial contains sterile bleomycin sulphate equivalent to 15 units or 30 units of bleomycin.
Sulfuric acid or Sodium hydroxide used, if necessary to adjust the pH.
Bleomycins are a group of related basic glycopeptides which differ in the terminal amine substituent of the common structural unit, bleomycin acid. The main components of Bleomycin for Injection are bleomycins A2 and B2. Chemically, bleomycin A2 is N1-[3-(dimethylsulfonio)propyl]-bleomycinamide and bleomycin B2 is N1-[4-(aminoiminomethyl)amino]butyl]-bleomycinamide.
The molecular formula of bleomycin A2 is C55H84N17O21S3 and a calculated molecular weight of 1414. The molecular formula of bleomycin B2 is C55H84N20O21S2 and a calculated molecular weight of 1425. The structural formula of bleomycins A2 and B2 are shown below.
Note: A unit of bleomycin is equal to the formerly used milligram activity. The term milligram activity is a misnomer and was changed to units to be more precise.
Resources
Didn’t find what you were looking for? Contact us.
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine.
*Speak with a Pfizer Medical Information Professional regarding your medical inquiry. Available 9AM-5PM ET Monday to Friday; excluding holidays.
Submit a medical question for Pfizer prescription products.
Report Adverse Event
Pfizer Safety
To report an adverse event related to the Pfizer-BioNTech COVID-19 Vaccine, and you are not part of a clinical trial* for this product, click the link below to submit your information:
Pfizer Safety Reporting Site*If you are involved in a clinical trial for this product, adverse events should be reported to your coordinating study site.
If you cannot use the above website, or would like to report an adverse event related to a different Pfizer product, please call Pfizer Safety at (800) 438-1985.
FDA Medwatch
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or call (800) 822-7967.