BICILLIN® L-A Description
(penicillin G benzathine)
Description
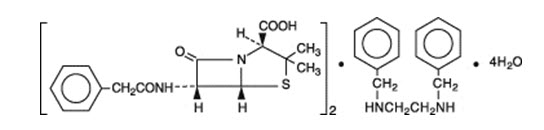
Bicillin L-A (penicillin G benzathine injectable suspension) is available for deep intramuscular injection. Penicillin G benzathine is prepared by the reaction of dibenzylethylene diamine with two molecules of penicillin G. It is chemically designated as (2S, 5R, 6R)-3,3-Dimethyl-7-oxo-6-(2-phenylacetamido)-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid compound with N,N' -dibenzylethylenediamine (2:1), tetrahydrate. It occurs as a white, crystalline powder and is very slightly soluble in water and sparingly soluble in alcohol. Its chemical structure is as follows:
Bicillin L-A contains penicillin G benzathine in aqueous suspension with sodium citrate buffer and, as w/v, approximately 0.65% sodium citrate, 0.59% povidone, 0.54% carboxymethylcellulose sodium, 0.53% lecithin, 0.12% methylparaben, and 0.013% propylparaben.
Bicillin L-A contains approximately 0.11 mEq of sodium per 600,000 units of penicillin G (approximately 2.59 mg of sodium per 600,000 units of penicillin G).
Bicillin L-A suspension in the disposable-syringe formulation is viscous and opaque. It is available in a 1 mL, 2 mL, and 4 mL sizes containing the equivalent of 600,000 (actual volume of 1.17 mL contains 620,100), 1,200,000 (actual volume of 2.34 mL contains 1,240,200), and 2,400,000 (actual volume of 4.67 mL contains 2,475,100) units respectively of penicillin G as the benzathine salt. Read CONTRAINDICATIONS, WARNINGS, PRECAUTIONS, and DOSAGE AND ADMINISTRATION sections prior to use.
Find BICILLIN® L-A medical information:
Find BICILLIN® L-A medical information:
BICILLIN® L-A Quick Finder
Health Professional Information
Description
Description
Bicillin L-A (penicillin G benzathine injectable suspension) is available for deep intramuscular injection. Penicillin G benzathine is prepared by the reaction of dibenzylethylene diamine with two molecules of penicillin G. It is chemically designated as (2S, 5R, 6R)-3,3-Dimethyl-7-oxo-6-(2-phenylacetamido)-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid compound with N,N' -dibenzylethylenediamine (2:1), tetrahydrate. It occurs as a white, crystalline powder and is very slightly soluble in water and sparingly soluble in alcohol. Its chemical structure is as follows:
Bicillin L-A contains penicillin G benzathine in aqueous suspension with sodium citrate buffer and, as w/v, approximately 0.65% sodium citrate, 0.59% povidone, 0.54% carboxymethylcellulose sodium, 0.53% lecithin, 0.12% methylparaben, and 0.013% propylparaben.
Bicillin L-A contains approximately 0.11 mEq of sodium per 600,000 units of penicillin G (approximately 2.59 mg of sodium per 600,000 units of penicillin G).
Bicillin L-A suspension in the disposable-syringe formulation is viscous and opaque. It is available in a 1 mL, 2 mL, and 4 mL sizes containing the equivalent of 600,000 (actual volume of 1.17 mL contains 620,100), 1,200,000 (actual volume of 2.34 mL contains 1,240,200), and 2,400,000 (actual volume of 4.67 mL contains 2,475,100) units respectively of penicillin G as the benzathine salt. Read CONTRAINDICATIONS, WARNINGS, PRECAUTIONS, and DOSAGE AND ADMINISTRATION sections prior to use.
Resources
Didn’t find what you were looking for? Contact us.
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine.
*Speak with a Pfizer Medical Information Professional regarding your medical inquiry. Available 9AM-5PM ET Monday to Friday; excluding holidays.
Submit a medical question for Pfizer prescription products.
Report Adverse Event
Pfizer Safety
To report an adverse event related to the Pfizer-BioNTech COVID-19 Vaccine, and you are not part of a clinical trial* for this product, click the link below to submit your information:
Pfizer Safety Reporting Site*If you are involved in a clinical trial for this product, adverse events should be reported to your coordinating study site.
If you cannot use the above website, or would like to report an adverse event related to a different Pfizer product, please call Pfizer Safety at (800) 438-1985.
FDA Medwatch
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or call (800) 822-7967.