AROMASIN® Clinical Studies
(exemestane)
14 CLINICAL STUDIES
14.1 Adjuvant Treatment in Early Breast Cancer
The Intergroup Exemestane Study 031 (IES) was a randomized, double-blind, multicenter, multinational study comparing exemestane (25 mg/day) vs. tamoxifen (20 or 30 mg/day) in postmenopausal women with early breast cancer. Patients who remained disease-free after receiving adjuvant tamoxifen therapy for 2 to 3 years were randomized to receive an additional 3 or 2 years of AROMASIN or tamoxifen to complete a total of 5 years of hormonal therapy.
The primary objective of the study was to determine whether, in terms of disease-free survival, it was more effective to switch to AROMASIN rather than continuing tamoxifen therapy for the remainder of five years. Disease-free survival was defined as the time from randomization to time of local or distant recurrence of breast cancer, contralateral invasive breast cancer, or death from any cause.
The secondary objectives were to compare the two regimens in terms of overall survival and long-term tolerability. Time to contralateral invasive breast cancer and distant recurrence-free survival were also evaluated.
A total of 4724 patients in the intent-to-treat (ITT) analysis were randomized to AROMASIN (exemestane tablets) 25 mg once daily (N = 2352) or to continue to receive tamoxifen once daily at the same dose received before randomization (N = 2372). Demographics and baseline tumor characteristics are presented in Table 5. Prior breast cancer therapy is summarized in Table 6.
| Parameter | Exemestane (N = 2352) | Tamoxifen (N = 2372) |
|---|---|---|
| Age (years): | ||
| Median age (range) | 63.0 (38.0 – 96.0) | 63.0 (31.0 – 90.0) |
| Race, n (%): | ||
| Caucasian | 2315 (98.4) | 2333 (98.4) |
| Hispanic | 13 (0.6) | 13 (0.5) |
| Asian | 10 (0.4) | 9 (0.4) |
| Black | 7 (0.3) | 10 (0.4) |
| Other/not reported | 7 (0.3) | 7 (0.3) |
| Nodal status, n (%): | ||
| Negative | 1217 (51.7) | 1228 (51.8) |
| Positive | 1051 (44.7) | 1044 (44.0) |
| 1–3 Positive nodes | 721 (30.7) | 708 (29.8) |
| 4–9 Positive nodes | 239 (10.2) | 244 (10.3) |
| >9 Positive nodes | 88 (3.7) | 86 (3.6) |
| Not reported | 3 (0.1) | 6 (0.3) |
| Unknown or missing | 84 (3.6) | 100 (4.2) |
| Histologic type, n (%): | ||
| Infiltrating ductal | 1777 (75.6) | 1830 (77.2) |
| Infiltrating lobular | 341 (14.5) | 321 (13.5) |
| Other | 231 (9.8) | 213 (9.0) |
| Unknown or missing | 3 (0.1) | 8 (0.3) |
| Receptor status*, n (%): | ||
| ER and PgR Positive | 1331 (56.6) | 1319 (55.6) |
| ER Positive and PgR Negative/Unknown | 677 (28.8) | 692 (29.2) |
| ER Unknown and PgR Positive†/Unknown | 288 (12.2) | 291 (12.3) |
| ER Negative and PgR Positive | 6 (0.3) | 7 (0.3) |
| ER Negative and PgR Negative/Unknown (none positive) | 48 (2.0) | 58 (2.4) |
| Missing | 2 (0.1) | 5 (0.2) |
| Tumor Size, n (%): | ||
| ≤ 0.5 cm | 58 (2.5) | 46 (1.9) |
| > 0.5 – 1.0 cm | 315 (13.4) | 302 (12.7) |
| > 1.0 – 2 cm | 1031 (43.8) | 1033 (43.5) |
| > 2.0 – 5.0 cm | 833 (35.4) | 883 (37.2) |
| > 5.0 cm | 62 (2.6) | 59 (2.5) |
| Not reported | 53 (2.3) | 49 (2.1) |
| Tumor Grade, n (%): | ||
| G1 | 397 (16.9) | 393 (16.6) |
| G2 | 977 (41.5) | 1007 (42.5) |
| G3 | 454 (19.3) | 428 (18.0) |
| G4 | 23 (1.0) | 19 (0.8) |
| Unknown/Not Assessed/Not reported | 501 (21.3) | 525 (22.1) |
| Parameter | Exemestane (N = 2352) | Tamoxifen (N = 2372) |
|---|---|---|
| ||
| Type of surgery, n (%): | ||
| Mastectomy | 1232 (52.4) | 1242 (52.4) |
| Breast-conserving | 1116 (47.4) | 1123 (47.3) |
| Unknown or missing | 4 (0.2) | 7 (0.3) |
| Radiotherapy to the breast, n (%): | ||
| Yes | 1524 (64.8) | 1523 (64.2) |
| No | 824 (35.5) | 843 (35.5) |
| Not reported | 4 (0.2) | 6 (0.3) |
| Prior therapy, n (%): | ||
| Chemotherapy | 774 (32.9) | 769 (32.4) |
| Hormone replacement therapy | 567 (24.1) | 561 (23.7) |
| Bisphosphonates | 43 (1.8) | 34 (1.4) |
| Duration of tamoxifen therapy at randomization (months): | ||
| Median (range) | 28.5 (15.8 – 52.2) | 28.4 (15.6 – 63.0) |
| Tamoxifen dose, n (%): | ||
| 20 mg | 2270 (96.5) | 2287 (96.4) |
| 30 mg* | 78 (3.3) | 75 (3.2) |
| Not reported | 4 (0.2) | 10 (0.4) |
After a median duration of therapy of 27 months and with a median follow-up of 34.5 months, 520 events were reported, 213 in the AROMASIN group and 307 in the tamoxifen group (Table 7).
| Event | First Events N (%) | |
|---|---|---|
| Exemestane (N = 2352) | Tamoxifen (N = 2372) | |
| Loco-regional recurrence | 34 (1.45) | 45 (1.90) |
| Distant recurrence | 126 (5.36) | 183 (7.72) |
| Second primary – contralateral breast cancer | 7 (0.30) | 25 (1.05) |
| Death – breast cancer | 1 (0.04) | 6 (0.25) |
| Death – other reason | 41 (1.74) | 43 (1.81) |
| Death – missing/unknown | 3 (0.13) | 5 (0.21) |
| Ipsilateral breast cancer | 1 (0.04) | 0 |
| Total number of events | 213 (9.06) | 307 (12.94) |
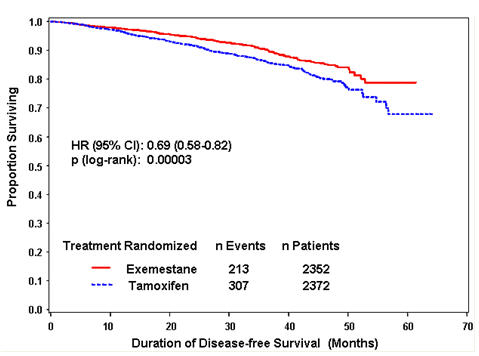
Disease-free survival in the intent-to-treat population was statistically significantly improved [Hazard Ratio (HR) = 0.69, 95% CI: 0.58, 0.82, P = 0.00003, Table 8, Figure 1] in the AROMASIN arm compared to the tamoxifen arm. In the hormone receptor-positive subpopulation representing about 85% of the trial patients, disease-free survival was also statistically significantly improved (HR = 0.65, 95% CI: 0.53, 0.79, P = 0.00001) in the AROMASIN arm compared to the tamoxifen arm. Consistent results were observed in the subgroups of patients with node negative or positive disease, and patients who had or had not received prior chemotherapy.
An overall survival update at 119 months median follow-up showed no significant difference between the two groups, with 467 deaths (19.9%) occurring in the AROMASIN group and 510 deaths (21.5%) in the tamoxifen group.
| ITT Population | Hazard Ratio (95% CI) | p-value (log-rank test) |
|---|---|---|
| ||
| Disease-free survival | 0.69 (0.58–0.82) | 0.00003 |
| Time to contralateral breast cancer | 0.32 (0.15–0.72) | 0.00340 |
| Distant recurrence-free survival | 0.74 (0.62–0.90) | 0.00207 |
| Overall survival | 0.91 (0.81–1.04) | 0.16* |
| ER and/or PgR positive | ||
| Disease-free survival | 0.65 (0.53–0.79) | 0.00001 |
| Time to contralateral breast cancer | 0.22 (0.08–0.57) | 0.00069 |
| Distant recurrence-free survival | 0.73 (0.59–0.90) | 0.00367 |
| Overall survival | 0.89 (0.78–1.02) | 0.09065* |
| Figure 1. Disease-Free Survival in the IES Study of Postmenopausal Women with Early Breast Cancer (ITT Population) |
 |
14.2 Treatment of Advanced Breast Cancer
Exemestane 25 mg administered once daily was evaluated in a randomized double-blind, multicenter, multinational comparative study and in two multicenter single-arm studies of postmenopausal women with advanced breast cancer who had disease progression after treatment with tamoxifen for metastatic disease or as adjuvant therapy. Some patients also have received prior cytotoxic therapy, either as adjuvant treatment or for metastatic disease.
The primary purpose of the three studies was evaluation of objective response rate (complete response [CR] and partial response [PR]). Time to tumor progression and overall survival were also assessed in the comparative trial. Response rates were assessed based on World Health Organization (WHO) criteria, and in the comparative study, were submitted to an external review committee that was blinded to patient treatment. In the comparative study, 769 patients were randomized to receive AROMASIN (exemestane tablets) 25 mg once daily (N = 366) or megestrol acetate 40 mg four times daily (N = 403). Demographics and baseline characteristics are presented in Table 9.
| Parameter | AROMASIN (N = 366) | Megestrol Acetate (N = 403) |
|---|---|---|
| Median Age (range) | 65 (35–89) | 65 (30–91) |
| ECOG Performance Status | ||
| 0 | 167 (46%) | 187 (46%) |
| 1 | 162 (44%) | 172 (43%) |
| 2 | 34 (9%) | 42 (10%) |
| Receptor Status | ||
| ER and/or PgR + | 246 (67%) | 274 (68%) |
| ER and PgR unknown | 116 (32%) | 128 (32%) |
| Responders to prior tamoxifen | 68 (19%) | 85 (21%) |
| NE for response to prior tamoxifen | 46 (13%) | 41 (10%) |
| Site of Metastasis | ||
| Visceral ± other sites | 207 (57%) | 239 (59%) |
| Bone only | 61 (17%) | 73 (18%) |
| Soft tissue only | 54 (15%) | 51 (13%) |
| Bone & soft tissue | 43 (12%) | 38 (9%) |
| Measurable Disease | 287 (78%) | 314 (78%) |
| Prior Tamoxifen Therapy | ||
| Adjuvant or Neoadjuvant | 145 (40%) | 152 (38%) |
| Advanced Disease, Outcome | ||
| CR, PR, or SD ≥ 6 months | 179 (49%) | 210 (52%) |
| SD < 6 months, PD or NE | 42 (12%) | 41 (10%) |
| Prior Chemotherapy | ||
| For advanced disease ± adjuvant | 58 (16%) | 67 (17%) |
| Adjuvant only | 104 (28%) | 108 (27%) |
| No chemotherapy | 203 (56%) | 226 (56%) |
The efficacy results from the comparative study are shown in Table 10. The objective response rates observed in the two treatment arms showed that AROMASIN was not different from megestrol acetate. Response rates for AROMASIN from the two single-arm trials were 23.4% and 28.1%.
| Response Characteristics | AROMASIN (N=366) | Megestrol Acetate (N=403) |
|---|---|---|
| Abbreviations: CR = complete response, PR = partial response, SD = stable disease (no change), TTP = time to tumor progression, C.I. = confidence interval, MA = megestrol acetate, AR = AROMASIN | ||
| Objective Response Rate = CR + PR (%) | 15.0 | 12.4 |
| Difference in Response Rate (AR-MA) | 2.6 | |
| 95% C.I. | 7.5, -2.3 | |
| CR (%) | 2.2 | 1.2 |
| PR (%) | 12.8 | 11.2 |
| SD ≥ 24 Weeks (%) | 21.3 | 21.1 |
| Median Duration of Response (weeks) | 76.1 | 71.0 |
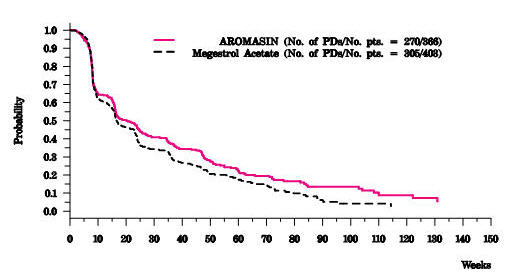
| Median TTP (weeks) | 20.3 | 16.6 |
| Hazard Ratio (AR-MA) | 0.84 | |
There were too few deaths occurring across treatment groups to draw conclusions on overall survival differences. The Kaplan-Meier curve for time to tumor progression in the comparative study is shown in Figure 2.
 |
Find AROMASIN® medical information:
Find AROMASIN® medical information:
AROMASIN® Quick Finder
Health Professional Information
Clinical Studies
14 CLINICAL STUDIES
14.1 Adjuvant Treatment in Early Breast Cancer
The Intergroup Exemestane Study 031 (IES) was a randomized, double-blind, multicenter, multinational study comparing exemestane (25 mg/day) vs. tamoxifen (20 or 30 mg/day) in postmenopausal women with early breast cancer. Patients who remained disease-free after receiving adjuvant tamoxifen therapy for 2 to 3 years were randomized to receive an additional 3 or 2 years of AROMASIN or tamoxifen to complete a total of 5 years of hormonal therapy.
The primary objective of the study was to determine whether, in terms of disease-free survival, it was more effective to switch to AROMASIN rather than continuing tamoxifen therapy for the remainder of five years. Disease-free survival was defined as the time from randomization to time of local or distant recurrence of breast cancer, contralateral invasive breast cancer, or death from any cause.
The secondary objectives were to compare the two regimens in terms of overall survival and long-term tolerability. Time to contralateral invasive breast cancer and distant recurrence-free survival were also evaluated.
A total of 4724 patients in the intent-to-treat (ITT) analysis were randomized to AROMASIN (exemestane tablets) 25 mg once daily (N = 2352) or to continue to receive tamoxifen once daily at the same dose received before randomization (N = 2372). Demographics and baseline tumor characteristics are presented in Table 5. Prior breast cancer therapy is summarized in Table 6.
| Parameter | Exemestane (N = 2352) | Tamoxifen (N = 2372) |
|---|---|---|
| Age (years): | ||
| Median age (range) | 63.0 (38.0 – 96.0) | 63.0 (31.0 – 90.0) |
| Race, n (%): | ||
| Caucasian | 2315 (98.4) | 2333 (98.4) |
| Hispanic | 13 (0.6) | 13 (0.5) |
| Asian | 10 (0.4) | 9 (0.4) |
| Black | 7 (0.3) | 10 (0.4) |
| Other/not reported | 7 (0.3) | 7 (0.3) |
| Nodal status, n (%): | ||
| Negative | 1217 (51.7) | 1228 (51.8) |
| Positive | 1051 (44.7) | 1044 (44.0) |
| 1–3 Positive nodes | 721 (30.7) | 708 (29.8) |
| 4–9 Positive nodes | 239 (10.2) | 244 (10.3) |
| >9 Positive nodes | 88 (3.7) | 86 (3.6) |
| Not reported | 3 (0.1) | 6 (0.3) |
| Unknown or missing | 84 (3.6) | 100 (4.2) |
| Histologic type, n (%): | ||
| Infiltrating ductal | 1777 (75.6) | 1830 (77.2) |
| Infiltrating lobular | 341 (14.5) | 321 (13.5) |
| Other | 231 (9.8) | 213 (9.0) |
| Unknown or missing | 3 (0.1) | 8 (0.3) |
| Receptor status*, n (%): | ||
| ER and PgR Positive | 1331 (56.6) | 1319 (55.6) |
| ER Positive and PgR Negative/Unknown | 677 (28.8) | 692 (29.2) |
| ER Unknown and PgR Positive†/Unknown | 288 (12.2) | 291 (12.3) |
| ER Negative and PgR Positive | 6 (0.3) | 7 (0.3) |
| ER Negative and PgR Negative/Unknown (none positive) | 48 (2.0) | 58 (2.4) |
| Missing | 2 (0.1) | 5 (0.2) |
| Tumor Size, n (%): | ||
| ≤ 0.5 cm | 58 (2.5) | 46 (1.9) |
| > 0.5 – 1.0 cm | 315 (13.4) | 302 (12.7) |
| > 1.0 – 2 cm | 1031 (43.8) | 1033 (43.5) |
| > 2.0 – 5.0 cm | 833 (35.4) | 883 (37.2) |
| > 5.0 cm | 62 (2.6) | 59 (2.5) |
| Not reported | 53 (2.3) | 49 (2.1) |
| Tumor Grade, n (%): | ||
| G1 | 397 (16.9) | 393 (16.6) |
| G2 | 977 (41.5) | 1007 (42.5) |
| G3 | 454 (19.3) | 428 (18.0) |
| G4 | 23 (1.0) | 19 (0.8) |
| Unknown/Not Assessed/Not reported | 501 (21.3) | 525 (22.1) |
| Parameter | Exemestane (N = 2352) | Tamoxifen (N = 2372) |
|---|---|---|
| ||
| Type of surgery, n (%): | ||
| Mastectomy | 1232 (52.4) | 1242 (52.4) |
| Breast-conserving | 1116 (47.4) | 1123 (47.3) |
| Unknown or missing | 4 (0.2) | 7 (0.3) |
| Radiotherapy to the breast, n (%): | ||
| Yes | 1524 (64.8) | 1523 (64.2) |
| No | 824 (35.5) | 843 (35.5) |
| Not reported | 4 (0.2) | 6 (0.3) |
| Prior therapy, n (%): | ||
| Chemotherapy | 774 (32.9) | 769 (32.4) |
| Hormone replacement therapy | 567 (24.1) | 561 (23.7) |
| Bisphosphonates | 43 (1.8) | 34 (1.4) |
| Duration of tamoxifen therapy at randomization (months): | ||
| Median (range) | 28.5 (15.8 – 52.2) | 28.4 (15.6 – 63.0) |
| Tamoxifen dose, n (%): | ||
| 20 mg | 2270 (96.5) | 2287 (96.4) |
| 30 mg* | 78 (3.3) | 75 (3.2) |
| Not reported | 4 (0.2) | 10 (0.4) |
After a median duration of therapy of 27 months and with a median follow-up of 34.5 months, 520 events were reported, 213 in the AROMASIN group and 307 in the tamoxifen group (Table 7).
| Event | First Events N (%) | |
|---|---|---|
| Exemestane (N = 2352) | Tamoxifen (N = 2372) | |
| Loco-regional recurrence | 34 (1.45) | 45 (1.90) |
| Distant recurrence | 126 (5.36) | 183 (7.72) |
| Second primary – contralateral breast cancer | 7 (0.30) | 25 (1.05) |
| Death – breast cancer | 1 (0.04) | 6 (0.25) |
| Death – other reason | 41 (1.74) | 43 (1.81) |
| Death – missing/unknown | 3 (0.13) | 5 (0.21) |
| Ipsilateral breast cancer | 1 (0.04) | 0 |
| Total number of events | 213 (9.06) | 307 (12.94) |
Disease-free survival in the intent-to-treat population was statistically significantly improved [Hazard Ratio (HR) = 0.69, 95% CI: 0.58, 0.82, P = 0.00003, Table 8, Figure 1] in the AROMASIN arm compared to the tamoxifen arm. In the hormone receptor-positive subpopulation representing about 85% of the trial patients, disease-free survival was also statistically significantly improved (HR = 0.65, 95% CI: 0.53, 0.79, P = 0.00001) in the AROMASIN arm compared to the tamoxifen arm. Consistent results were observed in the subgroups of patients with node negative or positive disease, and patients who had or had not received prior chemotherapy.
An overall survival update at 119 months median follow-up showed no significant difference between the two groups, with 467 deaths (19.9%) occurring in the AROMASIN group and 510 deaths (21.5%) in the tamoxifen group.
| ITT Population | Hazard Ratio (95% CI) | p-value (log-rank test) |
|---|---|---|
| ||
| Disease-free survival | 0.69 (0.58–0.82) | 0.00003 |
| Time to contralateral breast cancer | 0.32 (0.15–0.72) | 0.00340 |
| Distant recurrence-free survival | 0.74 (0.62–0.90) | 0.00207 |
| Overall survival | 0.91 (0.81–1.04) | 0.16* |
| ER and/or PgR positive | ||
| Disease-free survival | 0.65 (0.53–0.79) | 0.00001 |
| Time to contralateral breast cancer | 0.22 (0.08–0.57) | 0.00069 |
| Distant recurrence-free survival | 0.73 (0.59–0.90) | 0.00367 |
| Overall survival | 0.89 (0.78–1.02) | 0.09065* |
| Figure 1. Disease-Free Survival in the IES Study of Postmenopausal Women with Early Breast Cancer (ITT Population) |
 |
14.2 Treatment of Advanced Breast Cancer
Exemestane 25 mg administered once daily was evaluated in a randomized double-blind, multicenter, multinational comparative study and in two multicenter single-arm studies of postmenopausal women with advanced breast cancer who had disease progression after treatment with tamoxifen for metastatic disease or as adjuvant therapy. Some patients also have received prior cytotoxic therapy, either as adjuvant treatment or for metastatic disease.
The primary purpose of the three studies was evaluation of objective response rate (complete response [CR] and partial response [PR]). Time to tumor progression and overall survival were also assessed in the comparative trial. Response rates were assessed based on World Health Organization (WHO) criteria, and in the comparative study, were submitted to an external review committee that was blinded to patient treatment. In the comparative study, 769 patients were randomized to receive AROMASIN (exemestane tablets) 25 mg once daily (N = 366) or megestrol acetate 40 mg four times daily (N = 403). Demographics and baseline characteristics are presented in Table 9.
| Parameter | AROMASIN (N = 366) | Megestrol Acetate (N = 403) |
|---|---|---|
| Median Age (range) | 65 (35–89) | 65 (30–91) |
| ECOG Performance Status | ||
| 0 | 167 (46%) | 187 (46%) |
| 1 | 162 (44%) | 172 (43%) |
| 2 | 34 (9%) | 42 (10%) |
| Receptor Status | ||
| ER and/or PgR + | 246 (67%) | 274 (68%) |
| ER and PgR unknown | 116 (32%) | 128 (32%) |
| Responders to prior tamoxifen | 68 (19%) | 85 (21%) |
| NE for response to prior tamoxifen | 46 (13%) | 41 (10%) |
| Site of Metastasis | ||
| Visceral ± other sites | 207 (57%) | 239 (59%) |
| Bone only | 61 (17%) | 73 (18%) |
| Soft tissue only | 54 (15%) | 51 (13%) |
| Bone & soft tissue | 43 (12%) | 38 (9%) |
| Measurable Disease | 287 (78%) | 314 (78%) |
| Prior Tamoxifen Therapy | ||
| Adjuvant or Neoadjuvant | 145 (40%) | 152 (38%) |
| Advanced Disease, Outcome | ||
| CR, PR, or SD ≥ 6 months | 179 (49%) | 210 (52%) |
| SD < 6 months, PD or NE | 42 (12%) | 41 (10%) |
| Prior Chemotherapy | ||
| For advanced disease ± adjuvant | 58 (16%) | 67 (17%) |
| Adjuvant only | 104 (28%) | 108 (27%) |
| No chemotherapy | 203 (56%) | 226 (56%) |
The efficacy results from the comparative study are shown in Table 10. The objective response rates observed in the two treatment arms showed that AROMASIN was not different from megestrol acetate. Response rates for AROMASIN from the two single-arm trials were 23.4% and 28.1%.
| Response Characteristics | AROMASIN (N=366) | Megestrol Acetate (N=403) |
|---|---|---|
| Abbreviations: CR = complete response, PR = partial response, SD = stable disease (no change), TTP = time to tumor progression, C.I. = confidence interval, MA = megestrol acetate, AR = AROMASIN | ||
| Objective Response Rate = CR + PR (%) | 15.0 | 12.4 |
| Difference in Response Rate (AR-MA) | 2.6 | |
| 95% C.I. | 7.5, -2.3 | |
| CR (%) | 2.2 | 1.2 |
| PR (%) | 12.8 | 11.2 |
| SD ≥ 24 Weeks (%) | 21.3 | 21.1 |
| Median Duration of Response (weeks) | 76.1 | 71.0 |
| Median TTP (weeks) | 20.3 | 16.6 |
| Hazard Ratio (AR-MA) | 0.84 | |
There were too few deaths occurring across treatment groups to draw conclusions on overall survival differences. The Kaplan-Meier curve for time to tumor progression in the comparative study is shown in Figure 2.
 |
Health Professional Information
{{section_name_patient}}
{{section_body_html_patient}}
Resources
Didn’t find what you were looking for? Contact us.
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine.
*Speak with a Pfizer Medical Information Professional regarding your medical inquiry. Available 9AM-5PM ET Monday to Friday; excluding holidays.
Submit a medical question for Pfizer prescription products.
Report Adverse Event
Pfizer Safety
To report an adverse event related to the Pfizer-BioNTech COVID-19 Vaccine, and you are not part of a clinical trial* for this product, click the link below to submit your information:
Pfizer Safety Reporting Site*If you are involved in a clinical trial for this product, adverse events should be reported to your coordinating study site.
If you cannot use the above website, or would like to report an adverse event related to a different Pfizer product, please call Pfizer Safety at (800) 438-1985.
FDA Medwatch
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or call (800) 822-7967.