ABRYSVO® Dosage and Administration
(Respiratory Syncytial Virus Vaccine)
2 DOSAGE AND ADMINISTRATION
2.1 Dose and Schedule
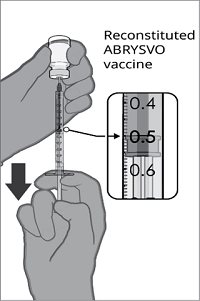
After reconstitution, a single dose of ABRYSVO is either approximately 0.5 mL (vial and prefilled syringe presentation) or 0.5 mL (vial and vial presentation) [see Dosage and Administration (2.2)].
2.2 Presentations and Reconstitution
ABRYSVO is supplied in 2 presentations as follows:
Vial and Prefilled Syringe Presentation
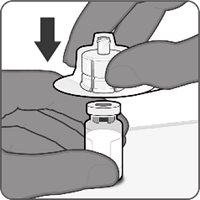
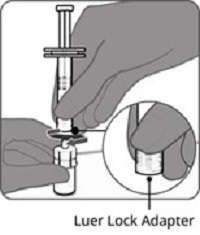
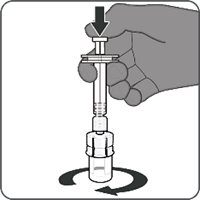
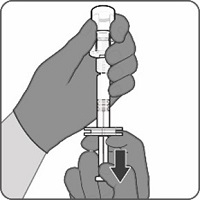
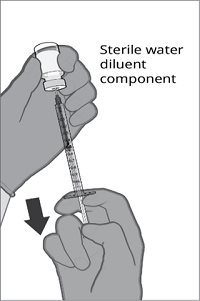
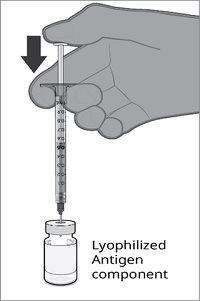
The vial and prefilled syringe presentation is supplied in a kit that includes a vial of Lyophilized Antigen Component (a sterile white powder), a prefilled syringe containing the Sterile Water Diluent Component, and a vial adapter.
Vial and Vial Presentation
The vial and vial presentation is supplied in cartons that include vials of Lyophilized Antigen Component (a sterile white powder) and vials containing the Sterile Water Diluent Component.
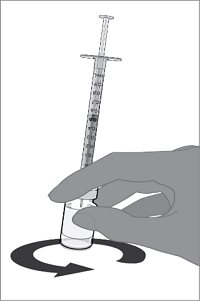
For both presentations, reconstitute the Lyophilized Antigen Component with the accompanying Sterile Water Diluent Component to form ABRYSVO, as described in the instructions below.
Reconstitution Instructions for Vial and Prefilled Syringe Presentation
Reconstitution Instructions for the Vial and Vial Presentation
2.3 Administration
For intramuscular injection
After reconstitution, ABRYSVO is a clear and colorless solution. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Discard if either condition is present.
Administer ABRYSVO immediately or store at room temperature [15°C to 30°C (59°F to 86°F)] and use within 4 hours. Discard reconstituted vaccine if not used within 4 hours.
Find ABRYSVO® medical information:
Find ABRYSVO® medical information:
ABRYSVO® Quick Finder
Health Professional Information
Dosage and Administration
2 DOSAGE AND ADMINISTRATION
2.1 Dose and Schedule
After reconstitution, a single dose of ABRYSVO is either approximately 0.5 mL (vial and prefilled syringe presentation) or 0.5 mL (vial and vial presentation) [see Dosage and Administration (2.2)].
2.2 Presentations and Reconstitution
ABRYSVO is supplied in 2 presentations as follows:
Vial and Prefilled Syringe Presentation
The vial and prefilled syringe presentation is supplied in a kit that includes a vial of Lyophilized Antigen Component (a sterile white powder), a prefilled syringe containing the Sterile Water Diluent Component, and a vial adapter.
Vial and Vial Presentation
The vial and vial presentation is supplied in cartons that include vials of Lyophilized Antigen Component (a sterile white powder) and vials containing the Sterile Water Diluent Component.
For both presentations, reconstitute the Lyophilized Antigen Component with the accompanying Sterile Water Diluent Component to form ABRYSVO, as described in the instructions below.
Reconstitution Instructions for Vial and Prefilled Syringe Presentation
Reconstitution Instructions for the Vial and Vial Presentation
2.3 Administration
For intramuscular injection
After reconstitution, ABRYSVO is a clear and colorless solution. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Discard if either condition is present.
Administer ABRYSVO immediately or store at room temperature [15°C to 30°C (59°F to 86°F)] and use within 4 hours. Discard reconstituted vaccine if not used within 4 hours.
Health Professional Information
{{section_name_patient}}
{{section_body_html_patient}}
Resources
Didn’t find what you were looking for? Contact us.
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine.
*Speak with a Pfizer Medical Information Professional regarding your medical inquiry. Available 9AM-5PM ET Monday to Friday; excluding holidays.
Submit a medical question for Pfizer prescription products.
Report Adverse Event
Pfizer Safety
To report an adverse event related to the Pfizer-BioNTech COVID-19 Vaccine, and you are not part of a clinical trial* for this product, click the link below to submit your information:
Pfizer Safety Reporting Site*If you are involved in a clinical trial for this product, adverse events should be reported to your coordinating study site.
If you cannot use the above website, or would like to report an adverse event related to a different Pfizer product, please call Pfizer Safety at (800) 438-1985.
FDA Medwatch
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or call (800) 822-7967.